Abstract
Background
Anastomotic leakages are severe complications of upper gastrointestinal surgery with serious morbidity and mortality. Until recently, only abscess drainage was possible. Since 2007, removable and repositionable covered metal self-expandable stents (RReCoMSeS) have been used in our hospital to cover leaks.
Methods
Patients with postsurgical gastrointestinal leaks treated with RReCoMSeS between January 2007 and March 2010 were retrospectively evaluated and described.
Results
Twenty-six patients were treated with RReCoMSeS (totally covered Choo/Hanaro and partially covered Endoflex stents). Included patients had anastomotic leaks after esophagectomy (15) and bariatric surgery (11). Overall successful sealing of the leak occurred in 81 % (including multiple procedures). In total 33 RReCoMSeS were used (mean 1.3 stents and 1.7 procedures per patient). Twenty-one of 33 RReCoMSeS succeeded in sealing the leak (64 %). Migration occurred in 24 % RReCoMSeS, and 9 % disintegrated. One stent (3 %) caused a perforation.
Conclusions
RReCoMSeS are a safe alternative for treating postsurgical leaks in the upper gastrointestinal tract. In 81 % of patients and with 64 % of the inserted stents, leaks were sealed successfully, with few complications. Fewer stents per patient were needed thanks to their repositionability. Stent migration is a major problem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
An anastomotic leak is a major complication after upper gastrointestinal surgery and a major source of mortality and morbidity. Anastomotic leakage in esophagectomy occurs in 4–30 % of cases. Mortality rates after major leakage are 70 % in older series and 35 % in more recent studies [1–5]. After bariatric surgery, anastomotic complications are found in 1–5 % of patients with a gastric bypass and 0.7–2.2 % after sleeve gastrectomy [6–9].
Until recently, the gold standard of treating anastomotic leaks was surgical or radiological drainage of the cavity or dismantling the anastomosis. However, these approaches have several disadvantages. Patients are in need of long-term parenteral nutrition and reoperative surgery in an already operated and infected area is difficult and may contribute to complications [3, 10]. In the past few years, an increasing number of small studies and case reports have described a novel endoscopic approach to anastomotic leaks: covered self-expandable stents. The endoscopically inserted covered self-expandable stent is an already well-established treatment modality in case of palliation of patients with malignant obstructions of the gastrointestinal tract (esophagus, duodenum, bile ducts, and colon) or treatment of benign esophageal strictures. The use of covered self-expandable stents for treatment of anastomotic leaks is still controversial, but more and more studies are reporting good results. Mainly the results of plastic types of stents are reported. Some small sample studies report up to 91 % success rates in acute leaks [1, 11, 12]. On the other hand, stenting has disadvantages as well, such as stent migration (3–40 %), obstruction (9–10 %), or disintegration [13–17].
Recently, repositionable and removable covered self-expandable metal stents (RReCoMSeS) have been developed and seem promising for temporary stenting of fistulae or leaks [1, 11–13, 17–21]. Our large teaching hospital is a referral center for esophageal and bariatric surgery and endoscopic mucosal resection in the esophagus. Since 2007, at least 50 self-expandable enteral and esophagus stents are placed per year in our endoscopy unit for both malignant and benign indications. Here we describe the results of RReCoMSeS in a specific subgroup of patients: anastomotic leakage after esophageal and bariatric surgery.
Methods
Patients
In this retrospective observational study, medical records were reviewed of patients treated with endoscopic removable and repositionable covered self-expandable metal stent placement in our hospital for anastomotic leakage, gastrointestinal perforation, or fistula formation after upper gastrological surgery between January 1, 2007, and March 1, 2010.
Methods and materials
Left cervical transhiatal esophagus resections were performed according to the latest surgical principles. A gastric tube was formed with a linear stapler (Gia, Covidien) resulting in a tube diameter of approximately 5 cm. Cervical anastomosis was formed by an end-to-end sutured technique. Bariatric surgery was performed laparoscopic according to the latest surgical principles; gastroenterostomy was formed by stapler technique (Endo-Gia, Covidien). In case of anastomotic leakage, wound drainage or radiological transcutaneous drainage was performed before endoscopic stenting procedure.
Endoscopic stenting was performed according to the following protocol. All patients receive monitored analgosedation with intravenous midazolam and/or fentanyl, and fluoroscopic guidance is used. The area of the fistula is identified by endoscopy and injection of the fistula with iodide-containing liquid contrast (20 ml of Iomeron 300, Bracco Imaging, Germany, mixed with 10 ml saline 0.9 %). The lesion is marked with a clip (Resolution, Boston Scientific), if necessary. Depending on the size of the fistula and local anatomy, the length of the RReCoMSeS is chosen (80 or 140 mm). The stent catheter is inserted in the upper gastrointestinal tract next to the endoscope and then released under direct endoscopic and fluoroscopic vision. In several patients, the stent was fixed by Resolution clips at the lower and upper margin, as indicated by the clinician’s personal experience.
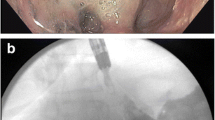
During the study period, three kinds of endoscopically removable stents were used. Originally, the totally silicon-covered Choo stent (M.I. Tech; 80 or 140 mm length, diameter 18 mm central and 24 mm at the margins of the stent) was used, later replaced by the Hanaro stent (also from M.I. Tech, same length and diameter, later also the colorectal Hanaro stent 60 mm long and of diameter 24 mm centrally and 30 mm at the margins) (Fig. 1). Two patients were treated with the partially silicon-covered Endoflex stent (Endotechniek; 80 or 140 mm length, 20 mm diameter).
Primary outcomes were successful stenting, complications of stenting, and fistula- or stent-related mortality. Success was defined as clinical and biochemical (C-reactive protein and leucocytes) normalization and absence of a lesion during stent extraction.
Results
Between January 1, 2007, and March 1, 2010, approximately 119 left cervical transhiatal esophagus resections for esophageal cancer were performed in our hospital. Bariatric surgery was performed in 574 patients in this period (gastric bypass in 262 and gastric sleeve resection in 312 patients). Anastomotic leakage occurred in 16 % of the esophageal resections and 4 % of the bariatric patients. Of these patients, 26 were found to be treated with RReCoMSeS. Fifteen patients were female (58 %) and 11 were male (42 %), with a mean age of 52.2 years (range, 29–78 years) (Table 1). Follow-up ranged from 2 to 144 weeks (mean, 56.4 weeks; median, 72 weeks).
Fifteen patients (58 %) were treated for an anastomotic leak after esophagectomy and subsequent gastric tube reconstruction with left cervical anastomosis in case of esophageal cancer. Twelve patients had a leakage of the cervical anastomosis and three patients had thoracic leaks. Cervical leaks were successfully closed in eight patients, although five stents migrated and one perforated. Thoracic leakages were all successfully treated and only one stent migrated.
Eleven patients (42 %) were treated with RReCoMSeS because of complications after bariatric surgery, 5 times after gastric bypass and 6 times after a gastric sleeve resection. Seven bariatric patients underwent a second bariatric surgery after unsuccessful previous bariatric surgery (gastric band or gastroplasty), and four had primary bariatric surgery. Of the gastric bypass patients, one was unsuccessfully treated, and two stents migrated and one disintegrated. Of the sleeve patients, one was unsuccessfully treated, and two stents disintegrated and one migrated.
Overall, 21 of 26 patients (81 %) had a successful sealing of the anastomotic leak after one or more stenting procedures. In three patients (11.5 %), no adequate sealing of the leak was achieved, in 1 patient (3.8 %) recurrent leakage was observed 1 week after stent removal, and 1 patient (3.8 %) had spontaneous retrograde oral evacuation of the stent after 1 day. Five patients (19 %) needed second or even third or fourth stent insertions as a result of migration or disintegration of the stent or persistent leak. Six patients (23 %) needed one or more attempts to reposition the stent before successful healing of the anastomotic leak was achieved.
In total, 33 RReCoMSeS were placed in 26 patients, with a mean of 1.3 RReCoMSeS per patient (range, 1–4) and 1.7 procedures per patient (range, 1–4). Twenty-one of the 33 RReCoMSeS were placed successfully with sealing of the leak (64 %). Migration occurred in 8 of 33 RReCoMSeS (24 %), and three stents (9 %) disintegrated. In 2 of 33 RReCoMSeS (6 %), anastomotic leakage persisted, and one stent (3 %) caused a secondary perforation. In 5 of the 13 previously described stent complications (migration, disintegration, persistent leak, and perforated stent), repositioning of the stent was performed, and 7 times, a new stent was placed. In both patients treated with an Endoflex stent, ingrowth of the stent was found to be a problem during stent removal.
Five patients died during the studied period (patients A, J, P, Q, and U), all after esophagectomy. The cause of death was not directly related to RReCoMSeS placement but was due to combinations of severe sepsis, kidney failure, and respiratory failure.
RReCoMSeS were removed in 21 patients (81 %) after a mean time of 11.4 weeks (range, 1–63 weeks). Other patients had their RReCoMSeS still in situ at time of inclusion or died before retrieval was possible. One patient had spontaneous oral evacuation of the stent after 1 day. In 2 of 33 RReCoMSeS (both Endoflex), retrieval was difficult because of tissue ingrowth.
The three patients (11.5 %) with disintegrating RReCoMSeS (at 31 [Hanaro] days and at 140 and 365 days [Choo]) had no complaints or signs of obstruction. They recovered without additional complications after endoscopic removal or rectal passage of all stent parts. One of these patients was lost to follow-up; therefore, no stent retrieval was performed.
Migration was the most frequent complication, observed more often in longer stents and stents with a smaller diameter. The 140 mm stents showed a migration rate of 33 %. No migration was observed in the partially covered stents (Endoflex), but only two of these were used. Best results with less migration were observed in de Hanaro 80 mm long, 24 mm wide colorectal type RReCoMSeS. Table 1 shows that using clips to fixate the stent to the adherent mucosa did not significantly prevent migration: 12 of 33 stents were clipped, of which five migrated (41 %), versus 21 of 33 that were not clipped, with only two migrations (9.5 %).
Discussion
In this report, we evaluate our experiences with RReCoMSeS in the treatment of anastomotic or staple line leakage after esophagectomy or bariatric surgery (gastric bypass or sleeve resection). During the study period, the anastomotic leakage rate in our hospital was 16 % after esophagectomy and 4 % after bariatric surgery, the latter mainly after revisions of earlier bariatric procedures. Both rates are comparable with those published in the literature; previous reports on the leakage rate after an esophagectomy or esophagogastrectomy showed up to 4–30 % anastomotic leaks, with a mortality rate of up to 35 % [1–5]. Leakage rates after bariatric surgery are reported to be 0.7–2.2 % after gastric sleeve resections and 1–5 % after gastric bypass procedures, with mortality up to 30 % [1–9, 22].
Before the introduction of RReCoMSeS in our hospital, leakage was treated with surgical drainage or eventually disconnection of the anastomosis resulting in extended surgical procedures. Since 2007, patients with upper gastrointestinal tract fistula or anastomotic leaks have been treated with RReCoMSeS in our gastroenterology department. This relatively large retrospective case series demonstrates that the use of RReCoMSeS is a feasible option for temporary stenting of postsurgical fistulae in the upper gastrointestinal tract, with low morbidity and mortality. However, repeat procedures remain a point of concern.
Overall, in 81 % of patients, RReCoMSeS were successful in covering the anastomotic or staple line leakage. In 23 % of patients, more than one procedure was necessary for repositioning of the stent or inserting a new stent before success, the result of migration, disintegration, or other causes. The mean number of procedures was 1.7 (range, 1–4). This result is comparable with the reported overall success rates of self-expandable plastic and metal stents are 90 and 62.5–100 %, respectively, until now, as shown in Table 2 [6, 12, 19, 23–26]. In case of leaks after esophagectomy, the following results have been reported (Table 2A). The largest study by Tuebergen et al. describes 32 patients with an intrathoracic esophageal anastomotic leak. Stenting with covered self-expandable metal stents resulted in a functional sealing in 78 % of the patients, and the method-related complications rate was 28 % [18]. Studies on stenting of leaks after bariatric surgery are scarce (Table 2B). The largest recent systematic review of Puli et al. [27] of seven studies, including 67 patients with leaks, reports an overall success and migration rate of 88 and 17 %, respectively. The results of all present studies with leaks after mixed types of surgery are listed in Table 2C. Swinnen et al. [20] retrospectively reviewed 88 self-expandable metal stent placements, demonstrating a success and migration rate of 84 and 11 %, respectively. In our study, the mean number stents used per patient was 1.3 (range, 1–4), which is better than the previously reported 1.8 to 2.0 stents per patients [6, 23, 25]. This may be due to the repositionable character of the RReCoMSeS. Overall, all mentioned studies in Table 2 show relatively small series; the largest includes 31 patients. Often patient groups are inhomogeneous, and different stents were used. Only one case report and no series have been reported on the use of RReCoMSeS [28].
One of the main problems of our series is the high migration rate: 8 of 33 RReCoMSeS migrated (24 %). This may be explained by the fact that these stents are used in a nonstenotic bowel segment and by the type of stent used. Choo and Hanaro stents are fully covered stents, without uncovered shoulders, which may lead to less grip on the mucosa. The fact that the partially uncovered Endoflex stent did not show any migration or leakage may demonstrate this. Other studies report comparable migration rates of 6–83 % for covered self-expandable plastic stents and 3–43 % for self-expandable metal stents [14, 16]. Stent migration thus appears to be an important problem in all studies about coverage of anastomotic leakages or fistulae. Fixation of the RReCoMSeS by clipping the margins did not have a significant effect on migration rates in our study, in contrary to the report of Vanbiervliet et al. [29]. Possible solutions for prevention of stent migration in nonstenotic disease maybe the use of large-diameter stents, such as the 24/30 mm diameter colorectal stents as described in patients D, M, U, V, and Y. The length of the stent could also have effect on easy migration. Peristaltic movements of the bowel may have more grip on larger stents. At this moment, new stent types, such as specific postbariatric stents, partially covered stents, and large-diameter stents, are being tested.
RReCoMSeS did not result in any life-threatening complications. All five deaths were not directly related to the stent or the inserting procedure but were caused by pneumonia or cancer-related pathology.
Three cases are particularly interesting because of disintegration of the stent. Fortunately, in all cases the remaining stent parts passed the gastrointestinal tract without complications and were retrieved rectally or endoscopically removed. These cases emphasize the need for removal of the stent after some weeks to avoid late complications such as perforation or stent disintegration. However, no guidelines are yet available on the removal interval after stenting in benign situations. Studies only report expert opinions that mention between 2 and 6 weeks [11, 20, 30]. In practice, we recently decided to remove stents after a maximum of 4 to 6 weeks to prevent stent ingrowth and/or disintegration.
Our study has the following limitations. First, this case series is retrospective, not randomized, and it describes patients with different types of previous surgery. Second, patient numbers are quite small. In addition, patients were treated with three different types of stents in several sizes. In some patients, stent-fixating clips were used, making the comparison between the described patients more difficult. This consecutive use of different stents and clips reflects the learning phase with this technique in this kind of benign, nonobstructive indication. However, our series reflects daily practice and is one of the larger reports describing the use of stents in anastomotic leakage in upper gastrointestinal surgery. Furthermore, to our knowledge, this study is the largest study describing the use of removable and repositionable stents.
In conclusion, the success rates of covering leakages with RReCoMSeS after surgery of the upper gastrointestinal tract are relatively high, and no severe complications were observed. Therefore, stenting should always be considered in patients with a postoperative or iatrogenic fistula in the upper gastrointestinal tract. Removable and repositionable stents such as RReCoMSeS are preferred because the number of stents required is decreased, thereby reducing the number of procedures and lowering costs. More experience should be developed in stent types that prevent migration, which seems to be the largest problem to attack.
References
Kauer WK, Stein HJ, Dittler HJ, Siewert JR (2008) Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc 22(1):50–53
Alanezi K, Urschel JD (2004) Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 10(2):71–75
Lerut T, Coosemans W, Decker G, De Leyn P, Nafteux P, van Raemdonck D (2002) Anastomotic complications after esophagectomy. Dig Surg 19(2):92–98
Whooley BP, Law S, Alexandrou A, Murthy SC, Wong J (2001) Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg 181(3):198–203
Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N (2001) Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg 88(10):1346–1351
Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, De la Torre RA, Thaler KJ, Miedema BW, Scott JS (2008) Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg 206(5):935–938
Brethauer SA, Hammel JP, Schauer PR (2009) Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 5(4):469–475
Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ (2008) Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 4(1):33–38
Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I, Shimonov M, Assalia A (2013) Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 27(1):240–245
Urschel JD (1995) Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 169(6):634–640
Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, Lippert H, Pross M (2005) Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc 61(7):891–896
Langer FB, Wenzl E, Prager G, Salat A, Miholic J, Mang T, Zacherl J (2005) Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg 79(2):398–403
Salminen P, Gullichsen R, Laine S (2009) Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc 23(7):1526–1530
Johnsson E, Lundell L, Liedman B (2005) Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esophagus 18(4):262–266
Lee BI, Choi KY, Kang HJ, Kim BW, Choi H, Kim CW, Jeong JJ, Park SH, Chung IS, Kim JJ, Park SM (2006) Sealing an extensive anastomotic leak after esophagojejunostomy with an antimigration-modified covered self-expanding metal stent. Gastrointest Endosc 64(6):1024–1026
Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje G, Herbold T, Hölscher AH, Metzger R (2009) Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 23(10):2258–2262
van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD (2011) Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 33(12):1292–1301
Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M (2008) Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg 12(7):1168–1176
Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM (2004) Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg 240(5):801–807
Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J (2011) Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc 73(5):890–899
Siersema PD, Homs MY, Haringsma J, Tilanus HW, Kuipers EJ (2003) Use of large-diameter metallic stents to seal traumatic nonmalignant perforations of the esophagus. Gastrointest Endosc 58(3):356–361
DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG (2002) Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 235(5):640–645
Eisendrath P, Cremer M, Himpens J, Cadière GB, Le Moine O, Devière J (2007) Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy 39(7):625–630
Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ (2010) Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg 89(3):931–936
Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hünerbein M (2009) Treatment of oesophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Br J Surg 96(8):887–891
Serra C, Baltasar A, Andreo L, Pérez N, Bou R, Bengochea M, Chisbert JJ (2007) Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg 17(7):866–872
Puli SR, Spofford IS, Thompson CC (2012) Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 75(2):287–293
Oshiro T, Kasama K, Umezawa A, Kanehira E, Kurokawa Y (2010) Successful management of refractory staple line leakage at the esophagogastric junction after a sleeve gastrectomy using the HANAROSTENT. Obesity Surg 20(4):530–534
Vanbiervliet G, Filippi J, Karimdjee BS, Venissac N, Iannelli A, Rahili A, Benizri E, Pop D, Staccini P, Tran A, Schneider S, Mouroux J, Gugenheim J, Benchimol D, Hébuterne X (2012) The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc 26(1):53–59
van Heel NC, Haringsma J, Wijnhoven BP, Kuipers EJ (2011) Endoscopic removal of self-expandable metal stents from the esophagus (with video). Gastrointest Endosc 74(1):44–50
Schweigert M, Dubecz A, Stadlhuber RJ, Muschweck H, Stein HJ (2011) Treatment of intrathoracic esophageal anastomotic leaks by means of endoscopic stent implantation. Interact Cardiovasc Thorac Surg 12(2):147–151
Freeman RK, Vyverberg A, Ascioti AJ (2011) Esophageal stent placement for the treatment of acute intrathoracic anastomotic leak after esophagectomy. Ann Thorac Surg 92(1):204–208
Salinas A, Baptista A, Santiago E, Antor M, Salinas H (2006) Self-expandable metal stents to treat gastric leaks. Surg Obes Relat Dis 2(5):570–572
Dai Y, Chopra SS, Kneif S, Hünerbein M (2011) Management of esophageal anastomotic leaks, perforations, and fistulae with self-expanding plastic stents. J Thorac Cardiovasc Surg 141(5):1213–1217
Feith M, Gillen S, Schuster T, Theisen J, Friess H, Gertler R (2011) Healing occurs in most patients that receive endoscopic stents for anastomotic leakage; dislocation remains a problem. Clin Gastroenterol Hepatol 9(3):202–210
D’Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS (2011) Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg 142(1):39–46
Acknowledgments
The authors thank E. J. Schoon, MD, PhD, and J. van Spreeuwel, MD, PhD, for their skillful endoscopic contribution.
Disclosures
The bariatric department of our hospital and Dr. F. J. Smulders received an education grant from Covidien for the year 2011. Drs. Stronkhorst, Nieuwenhuijzen, Gilissen, and Leenders have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leenders, B.J.M., Stronkhorst, A., Smulders, F.J. et al. Removable and repositionable covered metal self-expandable stents for leaks after upper gastrointestinal surgery: experiences in a tertiary referral hospital. Surg Endosc 27, 2751–2759 (2013). https://doi.org/10.1007/s00464-013-2802-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-2802-1