Abstract
Background
Transoral incisionless fundoplication (TIF), a novel endoscopic procedure for treating gastroesophageal reflux disease (GERD), currently is under evaluation. In case of treatment failure, subsequent revisional laparoscopic antireflux surgery (rLARS) may be required. This study aimed to evaluate the feasibility, safety, and outcomes of revisional antireflux surgery after previous endoscopic fundoplication.
Methods
Chronic GERD patients who underwent rLARS after a previous TIF procedure were included in the study. Pre- and postoperative assessment included GERD-related quality-of-life scores, proton pump inhibitor (PPI) usage, 24-h pH-metry, upper gastrointestinal endoscopy, and registration of adverse events.
Results
Revisional laparoscopic Nissen fundoplication was feasible for all 15 patients included in the study without conversions to open surgery. Acid exposure of the distal esophagus improved significantly after rLARS, and esophagitis, PPI usage, and hiatal hernia decreased. Quality of life did not improve significantly after rLARS, and 33 % of the patients experienced dysphagia.
Conclusion
Revisional laparoscopic Nissen fundoplication was feasible and safe after unsuccessful endoscopic fundoplication, resulting in objective reflux control at the cost of a relatively high rate of dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
At the beginning of this century, endoscopic procedures for the treatment of gastroesophageal reflux disease (GERD) were introduced as a less invasive alternative to laparoscopic antireflux surgery (LARS) [1]. Techniques included radiofrequency ablation, injection therapy, and suturing or stapling techniques [2–5]. The outcomes of endoscopic antireflux techniques, however, were disappointing, and many techniques have been abandoned. Of these techniques, the suturing and stapling techniques are the most similar to antireflux surgery because they attempt to reconstruct the anatomic reflux barrier at the gastroesophageal junction.
Transoral incisionless fundoplication (TIF), designed to resemble parts of the surgical fundoplication, currently is under evaluation. Although early reports showed encouraging outcomes, a number of early series resulted in reports of a substantial treatment failure rate [6–9].
In case of failure, subsequent revisional laparoscopic antireflux surgery (rLARS) would be an obvious next step in an attempt to control reflux in these patients. However, this could be technically more challenging due to the altered anatomic situation around the gastroesophageal junction induced by the endoscopic procedure and could lead to an increased rate of complications or suboptimal outcomes.
The current study aimed to evaluate the feasibility and safety of rLARS after previous TIF and to assess its efficacy in subjective and objective reflux control.
Methods
Patient characteristics
Patients who underwent rLARS after a previous TIF were included in the study. These patients were selected from a group prospectively followed in the context of clinical trials evaluating the TIF procedure at our institution between 2006 and 2008. Written informed consent was obtained from the patients, and the protocols were approved by the local medical ethics committee.
The TIF procedure was offered to GERD patients referred for surgical management by their gastroenterologists because they were refractory to antisecretory medication or dissatisfied with it. The inclusion criteria for the TIF procedure were chronic GERD (>6 months), age of 18 to 75 years, BMI lower than 36 kg/m2, and normal or hypotonic lower esophageal sphincter (LES) resting pressure (<30 mmHg).
The presence of gastroesophageal reflux was confirmed by pathologic 24-h esophageal pH monitoring. Patients were excluded from the study if they had a large hiatal hernia (>5 cm), Barrett’s esophagus, a hypertonic LES resting pressure, or motility disorders. In case of treatment failure after TIF, antisecretory medication was resumed on demand. The rLARS procedure was offered in case of continuing or recurrent typical GERD symptoms while the patient was receiving antisecretory medication in combination with anatomic wrap failure at endoscopy or pathologic pH measurements.
Procedure details
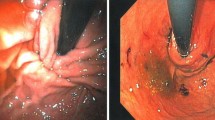
The TIF procedure was performed according to the TIF protocol previously described using the EsophyX-device (EndoGastric Solutions, Inc., Redmond) [10]. With the patient under general anesthesia and endotracheally intubated, a partial fundoplication was established endoscopically by performing sequential retractions of tissue and placement of multiple polypropylene H-fasteners.
The rLARS procedure included restoration of the pre-TIF anatomy by taking down the endoscopically created fundoplication via meticulous sharp dissection through the serosal adhesions and fasteners using endoscopic scissors. Subsequently, after mobilization of the fundus by dissection of two or three short gastric vessels, a “floppy” laparoscopic 360° Nissen fundoplication was performed by two experienced upper gastrointestinal (GI) surgeons. Adverse events during the surgery and postoperatively were recorded.
GERD monitoring
Objective and subjective GERD monitoring was performed at baseline and 3 to 6 months after each intervention (TIF and rLARS) according to protocol. At baseline (pre-TIF), after TIF, and after rLARS, symptomatic outcome was assessed using a GERD-related quality-of-life questionnaire (Health-Related Quality of Life [HRQL]) developed and validated to measure typical GERD symptoms in response to GERD therapy using a visual analog scale [11, 12]. General health condition satisfaction scores were monitored by the responses of patients as “satisfied,” “neutral,” or “dissatisfied.”
All the patients were advised to stop PPI usage 2 weeks after TIF or surgery and instructed to resume PPI usage in case of recurrent GERD-related symptoms. Their PPI usage was recorded in a drug diary. Objective assessment was performed by measurements of the distal esophageal acid exposure using the Orion II Ambulatory 24-h pH system (Medical Measurement Systems, Enschede, The Netherlands). A pH lower than 4 for up to 4.2 % of the monitoring time was considered physiologic esophageal acid exposure. Hiatal hernia, esophagitis (Los Angeles classification scale) and appearance of the fundoplication was evaluated by upper GI endoscopy at baseline and after each intervention.
Statistical analysis
Data distribution was evaluated using the Kolmogorov–Smirnov test. The acid exposure time and satisfaction rate were compared using the Wilcoxon signed rank test, and a paired Student’s t-test was used to compare GERD-HRQL scores. Statistical analysis was performed using Prism software version 5 (Graph Pad, San Diego, CA). Throughout this report, data are presented as mean ± standard error of the mean (SEM) or as median (interquartile range [IQR]) depending on data distribution. Differences were considered statistically significant at a p value lower than 0.05.
Results
At the time of data collection, 43 patients had undergone the TIF procedure at our institution in the context of clinical trials. Of these 43 patients, 38 had undergone the TIF1 procedure and 5 had undergone the TIF2 procedure in two different trials. Of the 15 patients who underwent rLARS, 14 had undergone a previous TIF1 procedure and 1 had undergone a previous TIF2 procedure.
The median age of the patients was 49 years (range 25–65 years), and seven of the patients were women. The mean interval between TIF and rLARS was 17 months (range 7–34 months), and mean follow-up period after rLARS was 14 months (range 3–34 months).
Patients, eligible for rLARS had continuing or recurrent GERD symptoms after TIF in combination with disruption of TIF fasteners (found in 76 %, with partial disruption in 53 % and complete disruption in 13 %) or pathologic acid exposure (found in 73 %).
The rLARS procedure was feasible for all the patients without the necessity of conversion to open surgery. The mean operation time was 112 min (range 57–206 min). The perioperative adverse events included a gastric perforation in one patient during dissection of the endoscopic fundoplication. The perforation was noted during the surgery and closed by endoscopic suturing. Postoperative upper GI radiography did not show leakage, and the event had no further consequences for the patient.
The major postoperative adverse event was dysphagia in 33 % of the patients, with 27 % needing one or more endoscopic pneumodilations. Other adverse events were mild and resolved for the majority of patients within the first week after rLARS.
The GERD-related quality-of-life scores after rLARS (18 ± 4) were improved significantly compared with baseline scores (32 ± 4) (p < 0.05), although the improvement between post-TIF and rLARS (23 ± 5) was not statistically significant (Fig. 1). The satisfaction score after surgery was significantly improved compared with the baseline score but did not differ from the score after TIF (Table 1).
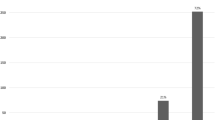
The median acid exposure time after rLARS (1.5; range 0.2–8.6) was significantly decreased compared with post-TIF (9.0; range 4.5–16.6) (p < 0.05) but not compared with baseline (7.1; range 5.9–12.8), and was normalized in 67 % of the patients (Fig. 2). After rLARS esophagitis, PPI usage and hiatal hernia improved (summary of outcomes is listed in Table 1).
Discussion
When medical therapy fails, surgery has been the only alternative for GERD patients. Laparoscopic Nissen fundoplication has become the gold standard, showing excellent outcomes in terms of GERD control [13]. Still, clinicians are reluctant to refer patients for surgery due to the invasiveness and complications such as dysphagia. Endoscopic fundoplication, introduced as an attractive minimally invasive alternative, is expected to have fewer side effects [14].
Unfortunately, in the early experience, endoscopic fundoplication did not control GERD satisfactorily in all patients. In fact, a substantial failure rate has been described [7, 8, 15]. Subsequently, rLARS would appear to be the next step for this group of patients. In this study, we report the outcomes of revisional surgery for unsuccessful previous TIF procedures in 15 patients.
In the majority of the patients included in this study, failure of the endoscopic fundoplication seemed to be caused by disruption of the polypropylene H-fasteners, which implies concerns about the durability of the endoscopically created fundoplication. Technically, rLARS after TIF was feasible for all the patients without conversions to open surgery. However, previous TIF showed a substantial change in anatomy, created with the TIF procedure, and with restoration of the “pre-TIF anatomic situation,” operation times were longer compared with primary LARS [16].
We encountered one gastric perforation as the only adverse event during surgery. The postoperative adverse events included a high rate of dysphagia (33 vs. 13 % after primary Nissen fundoplication) [16]. Dysphagia is difficult to cure and has, as shown in the current study, a serious impact on GERD-related quality of life. The question arises as to why the rate of dysphagia was high in this group.
Tension-free 360° Nissen fundoplications were performed by two experienced upper GI surgeons. We do not routinely use a bougie for calibration, and no bougies were used during the rLARS procedures. Besides restoration of the anatomy, the procedure did not differ from our standard primary LARS, after which dysphagia is uncommon. We take due care in creating a tension-free fundoplication by mobilizing the fundus along the greater curvature, about 10–15 cm inferior to the angle of His, with dissection of two to three short gastric vessels. After completion of the wrap, it is routinely checked for rotational tension by pulling the fundus through the retro-esophophafeal window to create a 360° wrap, which should stay in place after its release. A posterior crural repair is performed until the empty esophagus is just reached. The additional scar tissue at the fundus after the TIF procedure could perhaps be responsible for additional tension and therefore could be the cause for dysphagia.
Perhaps a bougie should have been be used in rLARS during wrap construction and crural closure. In a randomized controlled trial, Patterson et al. reported a significant decrease in postoperative dysphagia for patients who had a 56-Fr bougie placed during primary LARS compared with patients who had no bougie placed [17].
We speculate that a partial fundoplication such as the Toupet procedure may be a superior option for rLARS. Findings have shown the partial posterior fundoplication to be equally effective in reflux control, with less postoperative dysphagia in primary LARS [16]. On the other hand it is remarkable that revisional Nissen fundoplication after previous anti-reflux surgery does not result in a significantly increased rate of postoperative dysphagia (3–17 %) compared to primary LARS and results in improved GERD related quality of life scores [18–20].
For the 15 selected study patients, the pH measurements did not improve after TIF compared with baseline, and a slight increase in acid exposure of the distal esophagus is suggested (Fig. 2). A degradation of the antireflux barrier due to the endoscopic procedure also has been reported by other groups and ascribed to the learning curve for the TIF technique and the prototype device [8].
Currently, the TIF2 technique with the second-generation EsophyX device has been introduced and is suggested to improve outcomes [21]. The major difference between the initial TIF1 technique and TIF2 is the order and location of fastener placement, with the addition of rotational and longitudinal elements to an esophagogastric instead of a gastrogastric fundoplication [22]. Although acid exposure increased, GERD-related quality of life improved after TIF. This outcome confirms the poor correlation between symptoms and objective GERD testing and may be explained by a placebo effect at the time of data collection. In the experience with other early endoscopic GERD therapies, this effect was reported to be as high as 25–50 % [23, 24] .
After rLARS, acid exposure of the distal esophagus improved significantly, and esophagitis, PPI usage, and hiatal hernia decreased. Despite improved objective reflux control, the GERD-related quality of life did not improve significantly, nor did the general satisfaction scores, mainly due to the high rate of dysphagia, which is represented in the GERD-HRQL scores.
The single other study on this topic was published by Furnee et al. [25]. In their study, 11 patients underwent laparoscopic Nissen fundoplication after a failed TIF procedure. Gastric perforations occurred in 27 % of the patients, resulting in one conversion to laparotomy and a subphrenic abcess requiring additional surgical exploration in one patient. Reflux control after rLARS was satisfactory, but dysphagia after rLARS was high in their series as well (27 %).
The limitations of the current study were the small group of patients, the short follow-up period, and the selection bias due to enrollment of all the subjects from a group of highly selected patients in the context of clinical trials with the TIF procedure.
We conclude that revisional laparoscopic Nissen fundoplication is, although technically challenging, feasible and safe after failed endoscopic fundoplication. In this study, a significant improvement in objective reflux control was established at the cost of a relatively high rate of dysphagia, which had an impact on quality of life. How to prevent postoperative dysphagia after rLARS remains unclear and needs further research.
Abbreviations
- TIF:
-
Transoral incisionless fundoplication
- GERD:
-
Gastroesophageal reflux disease
- PPI:
-
Proton pump inhibitor
- rLARS:
-
Revisional laparoscopic anti-reflux surgery
References
Nason KS, Schuchert MJ, Witteman BP, Jobe BA (2008) Endoscopic therapies for the treatment of reflux disease. Semin Thorac Cardiovasc Surg 20:320–325
Schwartz MP, Wellink H, Gooszen HG, Conchillo JM, Samsom M, Smout AJ (2007) Endoscopic gastroplication for the treatment of gastro-oesophageal reflux disease: a randomised, sham-controlled trial. Gut 56:20–28
Rothstein R, Filipi C, Caca K et al (2006) Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: a randomized, sham-controlled trial. Gastroenterology 131:704–712
Corley DA, Katz P, Wo JM et al (2003) Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology 125:668–676
Deviere J, Costamagna G, Neuhaus H et al (2005) Nonresorbable copolymer implantation for gastroesophageal reflux disease: a randomized sham-controlled multicenter trial. Gastroenterology 128:532–540
Cadiere GB, Van Sante N, Graves JE, Gawlicka AK, Rajan A (2009) Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc 23:957–964
Hoppo T, Immanuel A, Schuchert M et al (2010) Transoral incisionless fundoplication 2.0 procedure using EsophyX for gastroesophageal reflux disease. J Gastrointest Surg 14:1895–1901
Repici A, Fumagalli U, Malesci A, Barbera R, Gambaro C, Rosati R (2010) Endoluminal fundoplication (ELF) for GERD using EsophyX: a 12-month follow-up in a single-center experience. J Gastrointest Surg 14:1–6
Witteman BP, Strijkers R, de Vries E et al (2012) Transoral incisionless fundoplication for treatment of gastroesophageal reflux disease in clinical practice. Surg Endosc 26(11):3307–3315
Cadiere GB, Buset M, Muls V et al (2008) Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg 32(8):1676–1688
Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA (1996) Quality-of-life scale for gastroesophageal reflux disease. J Am Coll Surg 183:217–224
Velanovich V (2007) The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 20:130–134
Dallemagne B, Weerts J, Markiewicz S et al (2006) Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 20:159–165
Cadiere GB, Buset M, Muls V et al (2008) Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg 32:1676–1688
Nieponice A, Jobe BA (2011) Endoscopic fundoplication: real or fantasy? J Gastrointest Surg 15(8):1295–1298
Broeders JA, Mauritz FA, Ahmed Ali U (2010) Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg 97:1318–1330
Patterson EJ, Herron DM, Hansen PD, Ramzi N, Standage BA, Swanström LL (2000) Effect of an esophageal bougie on the incidence of dysphagia following Nissen fundoplication: a prospective, blinded, randomized clinical trial. Arch Surg 135(9):1055–1061; discussion 1061–1062
Iqbal A, Awad Z, Simkins J et al (2006) Repair of 104 failed antireflux operations. Ann Surg 244:42–51
Curet MJ, Josloff RK, Schoeb O, Zucker KA (1999) Laparoscopic reoperation for failed antireflux procedures. Arch Surg 134:559–563
Floch NR, Hinder RA, Klingler PJ et al (1999) Is laparoscopic reoperation for failed antireflux surgery feasible? Arch Surg 134:733–737
Bell RC, Cadiere GB (2011) Transoral rotational esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc 25(7):2387–2399
Jobe BA, O’Rourke RW, McMahon BP et al (2008) Transoral endoscopic fundoplication in the treatment of gastroesophageal reflux disease: the anatomic and physiologic basis for reconstruction of the esophagogastric junction using a novel device. Ann Surg 248:69–76
Hogan WJ (2006) Clinical trials evaluating endoscopic GERD treatments: is it time for a moratorium on the clinical use of these procedures? Am J Gastroenterol 101:437–439
Pearl JP, Marks JM (2007) Endolumenal therapies for gastroesophageal reflux disease: are they dead? Surg Endosc 21:1–4
Furnee EJ, Broeders JA, Draaisma WA et al (2010) Laparoscopic Nissen fundoplication after failed EsophyX fundoplication. Br J Surg 97(5):1051–1055
Disclosures
Bart P. L. Witteman, Boudewijn F. Kessing, Gitte Snijders, Ger H. Koek, José M. Conchillo, and Nicole D. Bouvy have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witteman, B.P.L., Kessing, B.F., Snijders, G. et al. Revisional laparoscopic antireflux surgery after unsuccessful endoscopic fundoplication. Surg Endosc 27, 2231–2236 (2013). https://doi.org/10.1007/s00464-012-2685-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2685-6