Abstract
Background
Transoral incisionless fundoplication (TIF) treats gastroesophageal reflux disease (GERD) by creating a full-thickness esophagogastric plication using transmural fasteners. If unsuccessful, revision laparoscopic anti-reflux surgery (rLARS) may be performed. This study evaluated operative findings and clinical outcomes of rLARS in 28 patients with prior primary TIF.
Methods
Intraoperative findings, complications, and symptomatic outcomes with GERD health-related quality of life (GERD-HRQL) were evaluated prospectively in patients having rLARS after TIF. Results are median with interquartile range (IQR).
Results
Between 03/2009 and 08/2013, 28 patients underwent rLARS at 14 (13–50) months post-TIF for recurrent symptoms after initial improvement. Pre-rLARS endoscopies found hiatal hernia (9) and wrap disruption (12). All revisions were completed laparoscopically in 88 (70–90) min. Eight patients underwent partial fundoplication, the rest Nissen. No intraoperative or postoperative complications occurred. Operative findings included: No axial hernia in 65 %; Dense adhesions in 14 %; Fasteners incorporating the lateral crus in 95 %; Traction diverticuli from esophagus to crura in 21 %. Residual plication was noted anteriorly in 75 %, posteriorly in 0 %. Operative approaches: (1) Areas where the TIF fundoplication remained were left intact. This necessitated rolling the fundoplication over the fused area to prevent an endoscopic appearance of ‘fold’. (2) Fasteners were cut and left to migrate into the lumen, rather than being pulled out. (3) In 8 patients with fusion of the lateral crus to TIF fundoplication and no axial hernia, revision fundoplication was performed without mediastinal mobilization but with posterior hernia repair. One patient required subsequent surgery for small paraesophageal hernia, one for refractory gas-bloat after rLARS. Dysphagia in 2 patients resolved with dilation. GERD-HRQL improved from a median of 20 (8–27) pre-TIF and 10 (1–20) pre-rLARS to 3 (0–4) at 28 months (12–40) post-rLARS (p = 0.020 for pre-rLARS to post-rLARS).
Conclusion
rLARS after TIF can be performed safely with excellent clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Traditional surgical treatment of gastroesophageal reflux disease (GERD) has been supplemented by various transoral endoscopic procedures. To date none of these procedures have quite equaled the efficacy of laparoscopic fundoplication. When transoral procedures have been unsuccessful, patients have variously been offered medical therapy, a repeat transoral procedure, or revision to a laparoscopic fundoplication. This study focuses on the perioperative findings and outcomes of patients who underwent revision laparoscopic anti-reflux surgery (rLARS) of a prior transoral incisionless fundoplication (TIF) using the EsophyX device (EndoGastric Solutions, Inc., Redmond, WA, USA).
Although TIF has been found effective in patients with small hiatal hernia (≤2 cm), some patients will require additional surgery to relieve recurrent GERD symptoms and/or to resolve de novo hiatal hernia that was not recognized or developed over time. Little published data exists on the outcomes of patients who have undergone revision after TIF. A recent systematic review of 15 studies including 559 patients having TIF 1 or TIF 2 technique found clinical success (defined as cessation of PPI use) in 32–92 % of patients, with 7.2 % of the total study population undergoing revision to a laparoscopic fundoplication at a median of 9.5 months follow-up [1]. Reports of difficulty during or complications after revisional surgery have raised the question of whether a transoral fundoplication complicates subsequent laparoscopic fundoplication [2, 3].
The primary aim of this study was to describe the technical challenges seen by our center in performing rLARS. Additionally we evaluated the safety and clinical efficacy of laparoscopic revision of prior TIF fundoplication.
Patients and methods
Between March 2009 and August 2013 at our center, 165 patients with objective evidence of GERD (defined as abnormal pH testing or Los Angeles Grade B esophagitis) underwent primary TIF performed by the first author using the TIF 2.0 technique previously described [4]. Twenty-five patients (15 %) underwent rLARS. The three additional rLARS patients had their index TIF at outside facilities; 28 patients, therefore, comprised the study population. Three of these 28 (11 %) had concomitant laparoscopic hiatal repair with their primary TIF; the remainder (89 %) had a purely transoral procedure.
All revisions were completed laparoscopically. Short gastric vessels were divided as needed. Twenty patients (71 %) had a complete fundoplication; the other eight (29 %) had a partial fundoplication. The decision to perform a complete or partial fundoplication was based upon our experience with partial fundoplications that severe reflux is best treated with a complete fundoplication, but that less severe disease can be treated with a partial fundoplication with excellent clinical and objective results [5, 6]. Additionally, a partial fundoplication has frequently been associated with a lower incidence of severe bloating postoperatively, and concern about side effects was one of the major reasons patients elected to have the transoral fundoplication at their first surgery. All patients received perioperative antibiotic prophylaxis. Hiatal closure using 0-braided polyethylene suture was performed in 27 of 28 patients (96 %). Based on our own experience with failures of primary laparoscopic fundoplication (90 % involved hiatal failure) [7] and results from randomized controlled trials suggesting that mesh reinforcement leads to decreased recurrence rate [8–10], we elected to reinforce all of the hiatal repair with an acellular allograft mesh.
Patients undergoing laparoscopic revision of a prior TIF were elicited from a prospective database of patients undergoing laparoscopic anti-reflux procedures at a single institution. Institutional Review Board approval for the study was obtained. Patient demographics, time to revisional surgery, operative times, and certain operative details were collected prospectively. Retrospective review of operative notes, photographs, and videos was performed when needed. Postoperative complications and responses to the gastroesophageal reflux disease health-related quality of life (GERD-HRQL) questionnaire [11], and a standardized quality of life (QOL) tool used to evaluate GERD symptoms, were recorded. High-resolution impedance manometry and ambulatory pH testing off acid-suppressive medication was performed in all patients prior to rLARS.
The study endpoints included (1) Intraoperative findings, including density of adhesions, operative time, intraoperative complications; (2) Immediate postoperative outcomes including infections, length of stay, readmissions, or other interventions within the first 30 days of revisional surgery; (3) Longer-term outcomes determined by QOL responses.
Statistical analysis
All statistical analyses were performed using JMP version 10 (SAS Institute Inc., Cary, NC, USA). Continuous variables are reported using median and interquartile range (IQR) if the data were not distributed normally; mean and standard deviation are used for the data points with normal distribution. Categorical variables are presented as frequencies (%). The non-parametric Wilcoxon signed rank test was used to compare GERD-HRQL scores at presentation and after TIF and rLARS.
Results
The mean age at the time of rLARS was 55 years (±13.4) and 12 of 28 (43 %) of patients were male. Median follow-up was 14 (IQR = 7–38) months. Of all 28 patients who underwent rLARS, 20 (71 %) had undergone the initial TIF primarily for typical GERD symptoms (heartburn and/or regurgitation). The remaining 8 patients (29 %) suffered from extra-esophageal symptoms. Twenty-three patients (82 %) reported improvement/resolution of their symptoms after the primary TIF, and then experienced symptom recurrence significant enough to motivate revision; the remainder saw no improvement after primary TIF. All patients demonstrated abnormal pH testing prior to revision (mean 8.6 ± 4.8 %; 24-h total acid exposure). Esophagogastroduodenoscopy (EGD) with conscious sedation was performed prior to the revisional surgery in 25 patients; 12 patients had evidence of fundoplication loosening without significant hiatal hernia; 9 had evidence of axial herniation >2 cm leading to complete wrap disruption; and in 4 patients the transoral fundoplication appeared intact.
Median duration of rLARS was 88 (IQR = 70–90) min. Estimated blood loss was a median of 5 (IQR = 5–100) cc. Twenty patients underwent complete fundoplication; 8 patients underwent partial fundoplication. No intraoperative complications or postoperative leaks or abscesses occurred. All patients were discharged by postoperative day 2.
Intraoperative observations
Review of the operative note, photographs, and videos of the rLARS procedure, when available, revealed the following:
-
(1)
Eighteen patients (64 %) had no axial hiatal hernia at surgery, including 2 patients with an axial hiatal hernia >2 cm at preoperative endoscopy.
-
(2)
After mobilization including division of the phrenoesophageal membrane, hiatal dimensions were a median of 4 (IQR = 3.5–4.5) cm anterior–posterior and 2 (IQR = 2–3) cm transverse. The anterior–posterior dimension of the hiatus was >4 cm in 50 % of patients.
-
(3)
Dense adhesions external to the fundoplication were present in 4 patients (14 %). The remaining patients demonstrated minimal adhesions, and the prior fundoplication could easily be identified and dissected from surrounding structures. There was no difference in preoperative presentation between those with and without dense adhesions.
-
(4)
Fasteners incorporating crural fibers on the left aspect of the hiatus were found in 27 of 28 (95 %) patients. With the TIF technique, it is possible to incorporate the crus into the fundoplication, as fasteners traverse the esophageal wall toward the fundus that has been apposed to the esophagus by the tissue mold. Occasionally fasteners in this location were accompanied by very dense adhesion of the fundoplication to the hiatus; these are included in the 14 % with dense adhesions reported above. In the majority of instances identification and cutting the fasteners with laparoscopic scissors allowed ready dissection of the fundoplication from the hiatus.
-
(5)
Traction diverticuli from esophagus to crura or diaphragm were found in 6 of 28 (21 %) patients. In all 6 patients we observed fasteners incorporating crural fibers. These diverticuli were a few millimeters in diameter and up to 5 mm in length.
-
(6)
Good residual anterior plication was noted in 21 of 28 (75 %). The fundoplication typically was 1–2 cm in length and extended to the anterior mid-aspect of the esophagus, but not more medially than this 12:00 position. Partially free fasteners (i.e., one leg embedded in the esophageal or gastric wall, the other leg not embedded in any tissue) were seen to a variable extent.
-
(7)
Poor or no residual posterior plication was observed in all patients.
-
(8)
Mediastinal dissection less than 7 cm above the diaphragm was performed in 20 (71 %) patients. Eight patients did not require any mediastinal dissection and the phrenoesophageal membrane was intentionally left intact. Intra-abdominal esophageal length >3 cm was confirmed by intraoperative endoscopy in all patients.
-
(9)
One patient with a preoperative BMI of 31 had a large Belsey fat pad that prevented anterior fasteners from bridging the space from esophagus to fundus.
Intraoperative technique considerations based upon findings
-
(1)
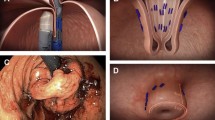
Areas where the fundus was fused to the esophagus were left intact. Early in our experience we folded the anterior fundus over the fused area. This led, however, to a fundoplication with an endoscopic appearance of ‘fold’. Although this ‘fold’ appearance did not have any clinical consequence, we subsequently learned to roll the fundoplication over the fused area, resulting in a more classic endoscopic appearance of the fundoplication (Fig. 1).
-
(2)
We avoided pulling fasteners out. Fasteners with one end free were cut and the intraluminal end was left in situ. Fasteners between a lumen and another structure (e.g., esophagus and crura) were gently displayed, then cut, and left to migrate into the lumen.
-
(3)
Traction diverticuli were clipped and divided if long, or divided and then imbricated with suture if short; the fastener trailing leg (if present) was left in the esophageal lumen (Fig. 2).
-
(4)
All patients with hiatal hernias underwent circumferential, Level 1 mediastinal dissection, and hiatal repair.
-
(5)
In 8 patients with good length, no significant hernia, and very dense adhesions of the lateral crus to TIF fundoplication, revisional surgery was performed without mediastinal mobilization, and the left-side crural/fundoplication fusion was left intact. Either posterior or anterior hiatal repair was still performed in 7 of these 8 patients, and then a laparoscopic fundoplication was created over the residual transoral fundoplication. These patients were no different in their pre-rLARS presentation than the remainder of the study group.
-
(6)
An air test, consisting of air insufflation in the esophageal/gastric lumen via the endoscope, with irrigant fluid covering the laparoscopic field, was used to look for any leaks. Radiographic examination was not performed prior to initiation of oral intake.
Follow-up
Two patients required subsequent laparoscopic surgery more than 30 days after the laparoscopic revision, one for a small paraesophageal hernia. The second patient developed refractory gas-bloat after rLARS with a complete fundoplication and requested revision to a partial fundoplication, with resolution of the gas-bloat. Two patients required postoperative dilation, after which their dysphagia resolved. No patients reported dysphagia in longer-term follow-up.
GERD-HRQL scores prior to index TIF, prior to rLARS, and post-rLARS were available in 17 of 28 (61 %) patients. The median GERD-HRQL score improved from 24 (IQR = 8–27) prior to TIF, to 10 (IQR = 1–20) prior to rLARS, to 3 (IQR = 0–4) at 28 months (IQR = 12–40) post-rLARS (Fig. 3).
Gastroesophageal reflux disease health-related quality of life (GERD-HRQL) scores at baseline, after transoral incisionless fundoplication (TIF) and after revision laparoscopic anti-reflux surgery (rLARS). Data are presented as median and interquartile range; p values were calculated using Wilcoxon sign-rank test
Discussion
The objectives of this study were to describe technical challenges in performing rLARS after TIF and secondly to evaluate the safety and clinical efficacy of laparoscopic revision of prior TIF fundoplication. We found that in all 28 patients, laparoscopic revision could be accomplished safely, that postoperative dysphagia was rare, and that QOL improved significantly after the revision.
To date, single-armed studies of TIF demonstrate less complete control of GERD than single-arm studies of laparoscopic fundoplication. A potential shorter recovery time and decrease in intensity and frequency of side effects after TIF have been suggested to counter-balance the lower success rate of TIF. Reported gas-bloat and dysphagia side effects appear to be lower after TIF than laparoscopic fundoplication, in fact almost non-existent [1, 12, 13]. In studies of the TIF 2 technique, 5–53 % of patients with recurrent reflux subsequently underwent revisional surgery, with an overall revision rate of 8.1 % at a median follow-up interval of 9.5 months [1, 14, 15]. In the current study, 25 (15 %) of our personal series of 165 patients having primary TIF subsequently underwent laparoscopic revisional surgery after a median of 14 (IQR = 7–38) months; the other 3 patients had primary TIF at other institutions. This percentage is greater than the 4 % revision rate we reported for patients having primary laparoscopic fundoplication with minimal hiatal hernia [7].
Perioperative complications, operative findings, and technique
One concern about TIF has been that laparoscopic revision to traditional fundoplication would be difficult or associated with significant complications. Furnee reported that of 11 patients having laparoscopic revision of prior TIF, intraoperative gastric perforation occurred in two patients, and one developed a subphrenic abscess after operation; one patient required open surgery; three had troublesome daily dysphagia [3]. Witteman reported 1 gastric perforation during laparoscopic revision of 15 patients having prior TIF [2]. Neither of these studies describes how fasteners were treated during rLARS. In the TIF procedure, multiple full-thickness fasteners traverse the esophagus and gastric fundus, leaving a potential for perforation during removal. Additionally, traction diverticuli of the esophagus can occur. If the fastener also traverses the crura or diaphragm, this increases tension on the lumen by adding a few millimeters of wall thickness, which, along with tension from respiratory excursion, can lead to traction diverticuli in the distal esophagus (in the worst case, and more acutely, esophageal perforation into the mediastinum or abdomen can result) [16]. With expectation of this potential for traction diverticuli and knowledge that the fasteners are full-thickness, the risk of perioperative leaks or abscesses can be minimized, we believe, by perioperative broad spectrum antibiotics, careful dissection, division but not removal of fasteners, and over-sewing of potential sources of leaks. In the current study and in a report by Perry of 7 patients having primary TIF and subsequent rLARS, no perioperative leaks or infectious complications occurred [17]. Dense adhesions of the fundus to surrounding structures were uncommon in our series; when they did occur it was primarily to the lateral crural fibers.
We did find the residual fundoplication to be densely fused to the esophagus. Based upon the limited extent of this residual fundoplication, potential risk to the patient of taking this residual down, and upon our experience that the transoral procedure creates a very symmetrical fundoplication, we did not try to take any residual fundus of the esophagus.
One interesting finding early in our experience was the potential for creating a fundoplication with the endoscopic appearance of a ‘fold’. We recognized this was due to folding, rather than rolling, the anterior fundus during creation of the anterior portion of the fundoplication (Fig. 4). Although this did not seem to have any clinical consequence, careful attention to the pre-existing fusion of fundus to esophagus, and intraoperative endoscopy, can avoid this odd appearance. Once we understood the principle of rolling the anterior fundus over any residual fundoplication, a classic anti-reflux valve was visualized endoscopically. There was no identifiable difference in clinical outcomes between patients with the appearance of ‘fold’ and those without.
A Cross section of esophagus (E), anterior (A) and posterior (P) gastric fundus, and typical start of finding tissue for anterior portion of fundoplication (red arrow). B Result of typical fundoplication. C Cross section illustrating area of fusion of fundus to esophagus after TIF (blue) and residual visible anterior fundoplication (green). The residual anterior is not fused, but can be overlooked when searching for fundus to fold anteriorly (red arrow). D Result is a fold in the fundoplication. Although this does not seem to be of any clinical consequence, the endoscopic appearance is different (Panels C and F). E Red arrow illustrates more appropriate choice of fundus to begin rolling anteriorly. F Result of rolling fundus anteriorly is a more typical appearance of fundoplication (Color figure online)
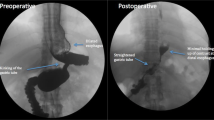
Operative findings to account for failure of the TIF in our study included posterior fundoplication disruption in most all patients, hiatal hernia in 1/3 of patients (axial length >2 cm or transverse diameter >3 cm), and anterior fundoplication disruption in 25 % of patients. In performing more traditional laparoscopic anti-reflux surgery, circumferential dissection of the esophagus includes division of the peritoneal reflection from the esophagus to the preaortic fascia. This retroesophageal window allows the fundus to be pulled posteriorly during a laparoscopic fundoplication. In the TIF 2 technique, the fundus is rotated posteriorly but is limited by an intact peritoneal reflection. Although 6–12 fasteners are deployed during TIF to create the posterior portion of the fundoplication, in no case did we observe residual fundoplication in this area during laparoscopic revision (Fig. 5). This suggests that the posterior portion of the TIF plication may be under tension, and brings into question the value of this part of the procedure. The anterior portion of the plication appeared intact in 75 % of patients. Early in our experience, when we performed laparoscopic visualization of creation of the fundoplication with TIF, we observed that the anterior movement of the fundus by the tissue mold is nearly identical to that seen during laparoscopic creation of a Dor anterior fundoplication. This may be the most tension-free aspect of the TIF procedure, and there may be opportunity here to increase the durability and/or extent of the anterior fundoplication as tension is one of the causes of fundoplication failure.
One-third of the patients in our series also had a hiatal hernia at re-operation. Upon review of the initial TIF procedure, it is clear in retrospect that at least 4 of these 10 patients had transverse hiatus >3 cm at the initial TIF.
In 95 % of the revisions, we found fastener deployment that incorporated or created dense adhesions of the crural fibers on the left side of the esophagus (3:00 on the clock face viewed anatomically in sagittal plane). In some instances this seemed to reinforce the phrenoesophageal membrane to such an extent that, in the absence of a hernia, it may be reasonable to leave this intact.
Outcomes
The outcome of patients having primary TIF and subsequent rLARS has also been a source of concern. In the same study by Furnee, 3 of 11 patients had ongoing troublesome daily dysphagia [3]. In Witteman’s study, 33 % of 15 patients reported dysphagia with 27 % requiring dilation [2]. There is no mention of the success of the dilations. QOL did not improve significantly after rLARS in that study. In contrast, only 2 patients (7 %) in current study required postoperative dilation. None of the patients reported dysphagia in longer-term follow-up. Quality of life measured by GERD-HRQL improved significantly after rLARS in our series. Although TIF creates certain technical challenges to revisional surgery, it does not appear that prior TIF increased re-operative complications in patients who go on to have rLARS.
This work has some limitations. First, although our database was prospectively maintained, we retrospectively reviewed operative notes, images, and videos. Second, the absence of post-rLARS pH testing prevented us from evaluating physiological outcomes post revision and comparing these outcomes versus outcomes of primary laparoscopic anti-reflux surgery. However, the primary goal of this study was not to assess objective outcomes of rLARS but to describe the challenges associated with performing rLARS after primary TIF. And finally, the possibility of selection bias (single center study) prior to initial TIF or rLARS may limit the generalizability of our findings.
Conclusion
Laparoscopic anti-reflux surgery after prior TIF fundoplication can be performed safely, with clinical outcomes comparable to primary anti-reflux surgery. Division without removal of full-thickness fasteners, anticipation of and proper treatment of small traction diverticuli, avoidance of unnecessary dissection, and endoscopic evaluation of the fundoplication resulted, in this series, in no perioperative complications, no long-term dysphagia, and normalization of quality of life.
References
Wendling MR, Melvin WS, Perry KA (2013) Impact of transoral incisionless fundoplication (TIF) on subjective and objective GERD indices: a systematic review of the published literature. Surg Endosc 27:3754–3761
Witteman BP, Kessing BF, Snijders G, Koek GH, Conchillo JM, Bouvy ND (2013) Revisional laparoscopic antireflux surgery after unsuccessful endoscopic fundoplication. Surg Endosc 27:2231–2236
Furnee EJ, Broeders JA, Draaisma WA, Schwartz MP, Hazebroek EJ, Smout AJ, van Rijn PJ, Broeders IA (2010) Laparoscopic Nissen fundoplication after failed EsophyX fundoplication. Br J Surg 97:1051–1055
Bell RC, Cadiere GB (2011) Transoral rotational esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc 25:2387–2399
Bell RC, Hanna P, Powers B, Sabel J, Hruza D (1996) Clinical and manometric results of laparoscopic partial (Toupet) and complete (Rosetti–Nissen) fundoplication. Surg Endosc 10:724–728
Bell RC, Hanna P, Mills MR, Bowrey D (1999) Patterns of success and failure with laparoscopic Toupet fundoplication. Surg Endosc 13:1189–1194
Bell RC, Fearon J, Freeman KD (2013) Allograft dermal matrix hiatoplasty during laparoscopic primary fundoplication, paraesophageal hernia repair, and reoperation for failed hiatal hernia repair. Surg Endosc 27:1997–2004
Oelschlager BK, Pellegrini CA, Hunter J, Soper N, Brunt M, Sheppard B, Jobe B, Polissar N, Mitsumori L, Nelson J, Swanstrom L (2006) Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 244:481–490
Frantzides CT, Madan AK, Carlson MA, Stavropoulos GP (2002) A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs. simple cruroplasty for large hiatal hernia. Arch Surg 137:649–652
Granderath FA, Schweiger UM, Kamolz T, Asche KU, Pointner R (2005) Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg 140:40–48
Velanovich V (1998) Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg 2:141–145
Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Langstrom G, Lind T, Lundell L, Collaborators LT (2011) Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305:1969–1977
Bell RC, Mavrelis PG, Barnes WE, Dargis D, Carter BJ, Hoddinott KM, Sewell RW, Trad KS, Da Costa Gill B, Ihde GM (2012) A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg 215:794–809
Wilson EB, Barnes WE, Mavrelis PG, Carter BJ, Bell RC, Sewell RW, Ihde GM, Dargis D, Hoddinott KM, Shughoury AB, Gill BD, Fox MA, Turgeon DG, Freeman KD, Gunsberger T, Hausmann MG, Leblanc KA, Deljkich E, Trad KS (2014) The effects of transoral incisionless fundoplication on chronic GERD patients: 12-month prospective multicenter experience. Surg Laparosc Endosc Percutan Tech 24:36–46
Hoppo T, Immanuel A, Schuchert M, Dubrava Z, Smith A, Nottle P, Watson DI, Jobe BA (2010) Transoral incisionless fundoplication 2.0 procedure using EsophyX for gastroesophageal reflux disease. J Gastrointest Surg 14:1895–1901
Bell RC, Freeman KD (2011) Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc 25:1975–1984
Perry KA, Linn JG, Eakin JL, Onders RP, Velanovich V, Melvin WS (2013) Transoral incisionless fundoplication does not significantly increase morbidity of subsequent laparoscopic Nissen fundoplication. J Laparoendosc Adv Surg Tech A 23:456–458
Disclosures
Dr. Reginald CW Bell, Dr. Ashwin A Kurian, and Ms. Katherine Freeman NP have no conflicts of interest or financial ties to disclose.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bell, R.C.W., Kurian, A.A. & Freeman, K.D. Laparoscopic anti-reflux revision surgery after transoral incisionless fundoplication is safe and effective. Surg Endosc 29, 1746–1752 (2015). https://doi.org/10.1007/s00464-014-3897-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3897-8