Abstract
Background
Two significant limitations of intraperitoneal drug therapy are limited drug distribution and poor penetration into peritoneal nodules. A possible solution is the application of the so-called “therapeutic pneumoperitoneum,” taking advantage of the gaseous nature and the pressure of capnoperitoneum during laparoscopy. Our objective was to develop a device able to apply such therapeutic pneumoperitoneum.
Methods
The technology presented here is a spraying device and can be introduced through a trocar. It is driven by mechanical pressure and consists of an injector, a line, and a nozzle. An in vivo experimental study was performed in five pigs. A transvaginal cholecystectomy was performed. At the end of the procedure, a standard dose of methylene blue was sprayed/infused into the abdominal cavity for 30 min (4 test animals w/therapeutic pneumoperitoneum (12 mmHg CO2) and 1 control animal w/conventional lavage (2 l intra-abdominal volume with extracorporeal circulation)). At the end of the procedure, all animals were autopsied and the peritoneum was analyzed. Outcome criteria were: (1) drug distribution (as assessed by the stained peritoneal surface at autopsy), and (2) diffusion into the peritoneum (presence or not of macroscopic staining of the outer aspect of the peritoneum immediately after surgery).
Results
Stained peritoneal surface was larger after aerosol application compared with peritoneal lavage, and staining more intense. Hidden peritoneal surfaces and the anterior abdominal wall were stained only in the aerosol group. In contrast to peritoneal lavage, the outer aspect of peritoneal membrane was immediately stained after pressurized spraying.
Conclusions
This device and the related approach significantly improve both distribution and penetration of a test substance into the peritoneal cavity in a large animal model. This might be a significant progress in treating intraperitoneal disease, in particular peritoneal carcinomatosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peritoneal involvement is a significant issue in the treatment of gastrointestinal and gynecological malignancies. For example, in gastric and ovarian cancer, more than 50% of patients will present with locoregional metastasis after “curative” surgical removal of the primary tumor. Unfortunately, there is no effective therapy to allow healing or even long-term palliation in this situation. The efficacy of intraperitoneal chemotherapy was demonstrated more than 20 years ago, as was the role of peritonectomy [1–3]. Recently, combination of complete cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to significantly improve survival in peritoneal carcinomatosis of colorectal origin [4]. Phase II trials of second-line intraperitoneal chemotherapy of ovarian cancer have shown the potential for patients to achieve complete response [5].
However, combined CRS and HIPEC are associated with morbidity of approximately 40% and mortality of 5% [6]. Moreover, efficacy of HIPEC is limited to patients with microscopic disease or tumor nodules smaller than 1–2 mm after CRS [7].
Intracavitary drug administration results in a much greater drug exposure for the cavity into which the agent is instilled compared with the plasma. The rationale of intraperitoneal drug administration is to increase the exposure of cancer cells within the peritoneal cavity to the drug while minimizing potential toxic effects to other organs. However, two significant pharmacokinetic problems limit the effectiveness of intraperitoneal chemotherapy: poor tumor penetration by the drug and incomplete irrigation of serosal surfaces by the drug-containing solution [8]. Thus, further progress in intraperitoneal chemotherapy will be due to better management of these pharmacokinetic problems.
In this respect, laparoscopic surgery offers an interesting opportunity: it takes place in a closed space—the peritoneal cavity, which is distended by the capnoperitoneum. Parameters, such as composition, temperature, pressure, and humidity, of the gas are well defined. The novel aspect is that these parameters could be steered to achieve intraoperative effects (such as cytotoxicity, prevention of surgical adhesions, antimicrobial effects, pain control, etc.) and in turn to improve postoperative outcome. Of course, such steering was impossible during conventional, “open” surgery.
We have developed a second-generation nebulizer that allows regional drug delivery into anatomical spaces, such as peritoneal or pleural cavity. The present experimental study presents the first results obtained with this nebulizer in the large animal model. Our general goal was to examine what happens within the abdominal cavity during nebulization of a vital stain (methylene blue). Our specific goals were to examine whether better stain distribution throughout the peritoneal cavity and better direct penetration of stain into peritoneum can be achieved compared with conventional peritoneal lavage under similar conditions.
Materials and methods
Study design
This is experimental in vivo study in the large animal model compared the effect of therapeutic capnoperitoneum (4 animals) with the effect of conventional lavage (1 control animal).
Nebulizer
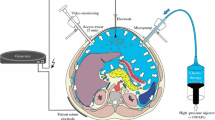
The nebulizer consists of several components, including an injector, a line, and a nozzle. The nozzle had a diameter of 0.2 mm and a pressure of approximately 8 bar was delivered upfront of the nozzle. The nebulizer was inserted through a 5-mm trocar and generated an aerosol within the abdominal cavity. The gaseous phase was consisting of CO2, the liquid phase of microparticles of methylene blue.
Animal model
After obtaining the authorization of the animal experiment review board of the State of Thüringen, we operated on five German female landrace pigs, weighing from 45 to 60 kg, under anesthesia using routine protocols. Pneumoperitoneum of 12 mmHg was established using a Veress needle. In the first step, a transvaginal cholecystectomy was performed in a three-trocar technique. In the second step, a peritoneal lavage or the application of therapeutic capnoperitoneum was performed according to the protocols below. The animals were euthanatized at the end of the procedure and immediately autopsied.
Experimental protocol
Similar protocols were applied for the control and test animals.
-
Control animal (n = 1) with conventional peritoneal lavage. Twenty milliliter methylene blue (1% Methylene blue VITIS®, Neopharma, Aschau i. Chiemgau, Germany) were diluted into 6 l of isotonic saline solution (0.9% NaCl). One inflow line and two outflow lines were placed into the abdomen, and the abdomen was filled with 2 l of solution. A continuous peritoneal lavage was maintained for 30 min with an inflow of 1 l/min over a rolling pump.
-
Test animals (n = 4) with therapeutic capnoperitoneum. Methylene blue (5 ml) was diluted into 10 ml of isotonic saline solution (0.9% NaCl). The nebulizer was inserted into a 5-mm trocar. The solution was nebulized by hand pressure at room temperature (20°C) over a few seconds into the abdominal cavity and an intra-abdominal pressure of 12 mmHg maintained for 30 min. The aerosol was eliminated using a pressure-limited waste system, including valve and filter (Fig. 1).
Results
Procedures could be performed as planned both in the test group (4 animals with nebulization) and in the control animal (conventional peritoneal lavage) and delivered the expected results.
The nozzle allowed rapid and effective nebulization of the methylene blue solution into the abdomen. Videoscopic control showed an immediate (within 5 s) staining of the complete abdominal cavity, including bowel loops, liver, anterior abdominal wall, and diaphragmatic peritoneum (Fig. 2). No droplet formation was observed and the surgeon’s view was not blurred. No hyperpressure developed in the abdominal cavity, as checked by the CO2 insufflator. The pressure-controlled valve of the waste filter did not open. No adverse cardiovascular or pulmonary effect was registered. No gas leak was detected during the experiment. At the end of the procedure, the aerosol could be completely collected into the waste filter.
Adequacy of stain distribution throughout the entire peritoneal cavity was clearly superior in the nebulization group compared with the control (Fig. 3). Immediate autopsy confirmed that staining of the serosal surfaces was better distributed after nebulization than after peritoneal lavage. In particular, hidden surfaces were stained in the nebulization group but not in the control animal; this was the case for anatomical structures located in the anterior, upper part of the abdomen (such as the abdominal wall).
Adequacy of stain distribution. Autopsy findings in the control (c1, c2) and test (t1, t2) animals. Staining of the serosal surfaces is better distributed after nebulization than after peritoneal lavage. Importantly, the hidden, anterior aspect of the stomach (*) is stained only in the nebulization group, as is the anterior abdominal wall (+)
Direct penetration of stain into the peritoneum was enhanced by nebulization (with the application of a pressure of 12 mmHg) compared with the conventional lavage (Fig. 4). Importantly, this difference was obtained despite the application of a higher (4×) total methylene blue doses in the control animal. In the nebulization group, the stain reached the backside of the peritoneum, as demonstrated by the staining of isolated retroperitoneal capillary vessels.
Direct penetration of stain into the peritoneum. Autopsy findings in the control (c) and test (t) animals. c1 and t1 show the front side of the peritoneum (towards the abdomen), c2 and t2 show the backside of the peritoneum. Almost no staining of the peritoneum is observed in the control group, despite the application of a higher (4×) methylene blue doses. In the nebulization group, staining has a patchy aspect (t1). Methylene blue staining has reached capillary vessels in the retroperitoneal fat tissue in the nebulization group (t2, arrow) but not in the control animal (c2, arrow)
Discussion
Approximately 10 years ago, we developed a micropump suitable for minimally invasive surgery procedures that allowed microdroplets of therapeutic substance to be distributed into the pneumoperitoneum (CO2), creating the so-called “therapeutic pneumoperitoneum,” or better said, the “therapeutic capnoperitoneum” [9]. The aerosol was produced by piezoelectric crystals stimulating three microperforated silicium chips. A feedback system regulated the amount of drug delivery depending on the effective gas flow. In vitro, the micropump was shown to be able to aerosolize various aqueous and ethanol solutions, including cytostatic and bacteriostatic drugs and adhesion-modulating agents. However, the function of the micropump was limited in vivo because of water condensation on the surface of the chips, so that further development was abandoned.
During the past 10 years, only few papers about laparoscopic nebulizers and their experimental or clinical applications were published. Alkhamesi et al. [10] showed that nebulized heparin and hyaluronic acid attenuates peritoneal tumor growth after laparoscopic surgery in a rodent mode. Sharon et al. [11] examined the effect of continuous intraabdominal nebulization of lidocaine during gynecological laparoscopic procedures in a limited number of patients. Druckrey-Fiskaaen et al. [12] examined laparoscopic spray application of fibrin sealant effects on hemodynamics and spray efficiency at various application pressures and distances. Recently, Greib et al. [13] compared various gas humidifying devices as a means of intraperitoneal local anesthetic administration, and only one of them was able to generate a therapeutic aerosol. However, the opportunities offered by a therapeutic capnoperitoneum were recognized as a potential “revolution in laparoscopic surgery” [14].
To our knowledge, no nebulizer has obtained so far EC or FDA certification so that it could be used for intraperitoneal drug application in the clinical setting. Moreover, no pharmacokinetics studies are available to prove that therapeutic capnoperitoneum improves drug distribution throughout peritoneal cavity or drug penetration into the tissues compared with peritoneal lavage.
This experimental study was designed to examine in the effect of nebulization of a vital stain into the abdominal cavity during laparoscopy. In particular, we wanted to determine distribution of methylene blue within the abdominal cavity and penetration of stain into the peritoneal layers. Current standard of care (peritoneal lavage) was used as a control.
Experimental data obtained in the animal model suggest limited exposure of the peritoneal surface during conventional peritoneal lavage. When peritoneal dialysis was performed in rodents with a solution containing methylene blue and bovine serum albumin, autopsy findings showed that large parts of the visceral and parietal peritoneum displayed no stain or very little stain [15]. In particular, the hidden aspects of the cecum and stomach as well as large portions of the small and large intestines and of the diaphragm remained unstained. Our results confirm this finding, namely that distribution of methylene blue within the peritoneal cavity is poor after peritoneal lavage.
Our results are encouraging, because they confirm that peritoneal nebulization allows better distribution of a substance throughout the abdominal cavity compared with conventional lavage. Immediate distribution of the stain all over the peritoneum was an impressive feature of the videoendoscopic monitoring during the procedure. It appears legitimate to extrapolate from our qualitative observations in a large animal model to the human setting. Unfortunately, quantitative data on the fraction of the peritoneum exposed to the lavage solution are not available in human patients. Moreover, functional peritoneal surface appear highly variable depending on the conditions [16]. To our knowledge, application of a therapeutic capnoperitoneum is one of the first concepts to increase exposure of the serosal surface and peritoneal tumor nodules. Such a therapeutic concept addresses an important medical need, because much of the residual tumor burden is probably untreated or undertreated by conventional techniques such as HIPEC.
Our data also show an advantage in direct penetration of stain into the normal peritoneum. Peritoneum is a single-layer cellular, mesothelial membrane that is supported by connective tissue. It offers protection against infection and tumor invasion [17]. However, in advanced cancer stages, tumor cells spread onto the peritoneal surface and into the surgical wound, and they develop macroscopic nodules up to several centimeters diameter. Experimental data show very limited penetration of drugs into the peritoneum and peritoneal nodules. Los et al. [18] measured platinum concentrations in CC531 colon adenocarcinoma growing intraperitoneally in the rat by proton-induced X-ray emission following intraperitoneal and intravenous administration of cisplatin. Tumor concentration was significantly elevated at 1.0 mm from the periphery but not at 1.5 mm. The same authors obtained similar results in tumor-bearing rats, in which the drug concentration was significantly elevated at a depth of 1 mm in the tumor nodules 24 h (single dose) or 48 h (three injections) after intraperitoneal administration [19]. Thus, the current principle is to treat the macroscopic (visible) malignant peritoneal disease with CRS and, immediately after, to treat the remaining microscopic (nonvisible) malignant peritoneal disease with HIPEC. However, efficacy of HIPEC remains hampered by the pharmacokinetics aspects defined above.
Dedrick and Flessner [8] has shown that limited direct penetration of drugs into tumor tissue remains an important practical and theoretical concern for regional drug therapy in the peritoneal cavity. The present experiment has shown that, after nebulization, methylene blue reaches the backside of the peritoneum and the capillary vessels located in the retroperitoneum. This is an exciting experimental finding, showing that nebulization of a substance into the abdominal cavity might be a significant progress to master this penetration problem and could help to improve clinical results of intraperitoneal drug therapy by allowing therapy of larger tumor nodules.
This study is a first step toward applying therapeutic capnoperitoneum with chemotherapeutic agents in the human patient. Some chemotherapeutic agents (such as doxorubicin) are approved for intraperitoneal use, so that regulatory framework conditions are rather favorable. However, four main problems remain to be addressed:
-
(1)
Drug uptake by capillary flow into the general circulation might increase systemic side-effects and paradoxically decrease the delivery of drug to the tumor. The fundamental goal of intraperitoneal chemotherapy is to increase exposure of the contents of the peritoneal cavity while reducing systemic toxic effects. On the one side, poor drug penetration limits cytotoxicity and tumor response. On the other side, it may protect sensitive normal cells, e.g., the mucosa of the gastrointestinal tract. Thus, increased drug distribution and tissue penetration (e.g., through nebulization) could result in an increased regional advantage but a higher systemic toxicity, because the gradient between peritoneal and plasma concentrations will narrow compared with conventional peritoneal lavage. However, we expect the gradient between local drug concentration in the peritoneal nodule and systemic compartment being much more favorable after application of the therapeutic capnoperitoneum into the abdomen than after intravenous administration.
A possible answer to address this question would be to apply the same dose intraperitoneally as during systemic chemotherapy. By definition, systemic uptake from the peritoneal cavity cannot be superior after intravenous, systemic administration, so that the safety profile would be favorable.
Until now, there has been a single experimental study to compare the pharmacokinetics of intraperitoneal ropivacaine—a local anesthetic—administered by instillation or nebulization in five pigs [20]. The pharmacokinetic profile of ropivacaine nebulization was found to be similar to direct intraperitoneal instillation, but with a lower absorption rate. This result supports further development of our concept of therapeutic capnoperitoneum.
-
(2)
An enhanced local drug delivery might be associated with specific locoregional toxic effects, such as abdominal pain, bowel perforation, anastomotic leakage, infection, or postoperative ileus, that need to be carefully evaluated. However, because the total drug dose (e.g., the same as for systemic chemotherapy) will be by far (a factor) less than for hyperthermic intraperitoneal chemotherapy (HIPEC), this problem is not expected to be limiting.
-
(3)
Administration of drugs via aerosolization can lead to measurable air concentrations in the breathing zone of workers providing treatment. Labor safety aspects of manipulating a toxic aerosol are significant and will be object of further studies. Our preliminary research has shown that appropriate technical solutions are available, which are routinely used in other applications, in particular in the automobile and chemical industry. These solutions include development of standard operating procedures (SOPs), training, health monitoring, room containment and remote control, negative pressure room ventilation, downward laminar air flow, use of respiratory protection and gloves, labeling, waste disposal, decontamination procedures, etc. These organizational and technical specifications, rules, and procedures will now be developed together with specialists from the industry to protect both the patient and the medical team from any harm and to meet regulatory requirements.
-
(4)
The micropump itself needs to receive CE and FDA approval as a medical device. Thus, further studies are necessary to define the optimal framework for applying this novel, promising therapeutic approach in the human patient. Next steps are ex vivo studies with chemotherapeutic agents (in particular doxorubicin, cisplatin, and oxaliplatin) on fresh peritoneal samples from patients suffering carcinomatosis, as well as CE certification as a medical device in Europe.
References
Markman M (1985) Intracavitary chemotherapy [review]. Crit Rev Oncol Hematol 3:205–233
Sugarbaker PH, Gianola FJ, Speyer JL, Wesley R, Barofsky I, Myers CE (1985) Prospective randomized trial of intravenous v. intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 12:101–111
Sugarbaker PH (1995) Peritonectomy procedures. Ann Surg 221:29–42
Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, Bonastre J (2009) Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27:681–685
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955
Maggiori L, Elias D (2010) Curative treatment of colorectal peritoneal carcinomatosis: current status and future trends [review]. Eur J Surg Oncol 36:599–603
Markman M (2003) Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol 4:277–283
Dedrick RL, Flessner MF (1997) Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 89:480–487
Reymond MA, Hu B, Garcia A, Reck T, Kockerling F, Hess J, Morel P (2000) Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc 14:51–55
Alkhamesi NA, Ridgway PF, Ramwell A, McCullough PW, Peck DH, Darzi AW (2009) Peritoneal nebulizer. A novel technique for delivering intraperitoneal therapeutics in laparoscopic surgery to prevent locoregional recurrence. Surg Endosc 19:1142–1146
Sharon A, Hirsh I, Kaufman Y, Ostrovski L, Brandes-Klein O, Spiegel D, Shenderey A, Lissak A (2008) The effect of continuous intraabdominal nebulization of lidocaine during gynecological laparoscopic procedures: a pilot study. Gynecol Surg 5:221–225
Druckrey-Fiskaaen KT, Janssen MW, Omidi L, Polze N, Kaisers U, Nur I, Goldberg E, Bokel G, Hauss J, Schön MR (2007) Laparoscopic spray application of fibrin sealant effects on hemodynamics and spray efficiency at various application pressures and distances. Surg Endosc 21:1750–1759
Greib N, Schlotterbeck H, Dow WA, Joshi GP, Geny B, Diemunsch PA (2008) An evaluation of gas humidifying devices as a means of intraperitoneal local anesthetic administration for laparoscopic surgery. Anesth Analg 107:549–551
Canis M, Matsuzaki S, Bourdel N, Jardon K, Cotte B, Botchorishvili R, Rabischong B, Mage G (2007) Peritoneum and laparoscopic environment [review]. Bull Cancer 94:1043–1051
Flessner MF (1996) Small-solute transport across specific peritoneal tissue surfaces in the rat. J Am Soc Nephrol 7:225–233
Steller MA, Egorin MJ, Trimble EL, Bartlett DL, Zuhowski EG, Alexander HR, Dedrick RL (1999) A pilot phase I trial of continuous hyperthermic peritoneal perfusion with high-dose carboplatin as primary treatment of patients with small-volume residual ovarian cancer. Cancer Chemother Pharmacol 43:106–114
Elias D, Goéré D (2007) Treat the peritoneum with respect! It’s our first line of defense against carcinomatosis. J Chir (Paris) 144:275–276
Los G, Mutsaers PH, van der Vijgh WJ, Baldew GS, de Graaf PW, McVie JG (1989) Direct diffusion of cis-diaminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 49:3380–3384
Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG (1990) Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol 25:389–394
Betton D, Greib N, Schlotterbeck H, Joshi GP, Ubeaud-Sequier G, Diemunsch P (2010) The pharmacokinetics of ropivacaine after intraperitoneal administration: instillation versus nebulization. Anesth Analg 111:1140–1145
Disclosures
This study was funded by Reger Medizintechnik GmbH, Rottweil, Germany. Alexander Hetzel is employed by and has an equity interest in Reger Medizintechnik. Wiebke Solaß, Giorgi Nadiradze, Emil Sagynaliev, and Marc A. Reymond have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solaß, W., Hetzel, A., Nadiradze, G. et al. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 26, 1849–1855 (2012). https://doi.org/10.1007/s00464-012-2148-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2148-0