Abstract
Background
The effects of conversion to open surgery during laparoscopic resection for colorectal cancer on long-term oncologic outcomes still are unclear.
Methods
All 450 laparoscopic colorectal resections for cancer performed at a single center between 1994 and 2008 and included in a prospectively maintained database were considered. Patients who required conversion to open surgery (CONV) were matched 1:2 with laparoscopically completed cases (LAP) and 1:5 with open surgery cases (OPEN) for age, American Society of Anesthesiologists (ASA) score, year of surgery, tumor location, and tumor stage. Fisher’s exact, chi-square, and Wilcoxon tests were used as appropriate. Kaplan–Meier curves were compared to analyze survival.
Results
In this study, 31 CONV cases were independently compared with 62 LAP and 155 OPEN cases. Compared with the LAP and OPEN patients, the CONV patients were characterized by a numerically higher rate of preoperative comorbidity (61.3% vs LAP, 51.6; P = 0.4 and OPEN, 48.4%; P = 0.2), male gender (77.4% vs LAP, 59.7%; P = 0.09 and OPEN, 58.1%; P = 0.05), and a significantly higher mean body mass index (29.6 vs LAP, 26.8; P = 0.012 and OPEN, 28.8; P = 0.3). The pathologic tumor stage, location, and chemotherapy and radiotherapy rates were comparable among the groups. After a median follow-up period of 4.1, 4.2, and 4.6 years, the 5-year disease-free survival rate was significantly lower for the CONV patients (40.2%) than for the LAP (70.7%, P = 0.01) or the OPEN (63.3%, P = 0.04) patients. However, the 5-year cancer-specific survival rates were similar among the CONV (94.4%), LAP (86.1%, P = 0.36), and OPEN (84.9%, P = 0.14) patients.
Conclusions
Conversion to open surgery does not affect oncologic outcomes, although CONV patients have increased comorbidity rates affecting long-term mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic resection is widely accepted in the treatment of colon cancer based on a number of prospective randomized trials [1–3]. However, the effects of conversion to open surgery on oncologic outcomes remain unclear [4]. In fact, the conclusions of the studies examining this issue are controversial for both colon [5–7] and rectal [8–10] carcinoma. The interpretation of these conflicting results is difficult because converted and laparoscopically completed cases often differ inherently based on patient characteristics, comorbidity status, and tumor stage [11]. In this respect, a case-matched study can minimize the risks of selection bias and allow a more accurate analysis of the oncologic outcomes when conversion to open surgery becomes necessary.
This study therefore aimed to compare the oncologic outcomes for patients who underwent converted laparoscopic colorectal resection with those for two separate control groups, namely, laparoscopically completed cases and open surgical procedures.
Methods
Patients undergoing laparoscopic colectomy or rectal resection for cancer between 1994 and March 2008 were identified through a prospectively maintained, institutional review board (IRB)-approved departmental database. The database includes demographic characteristics, patient comorbidities, intraoperative variables, and postoperative and long-term oncologic outcomes.
The laparoscopic operations were performed by eight different surgeons, whereas five additional surgeons performed only open colorectal resections. For the patients who continued postoperative cancer surveillance elsewhere, oncologic follow-up assessment was performed by phone interviews and through the request for clinical documentation. On the other hand, follow-up assessment at our institution was based on a combination of physical examination, serial evaluation of biochemical tumor markers, colonoscopy, and computed tomography (CT) scans, which varied slightly among individual surgeons but were all within accepted standards [12].
The exclusion criteria included stage 4 disease, urgent or palliative surgery, R2 resections, concurrent inflammatory bowel disease or hereditary cancer, and cases without follow-up data. Conversion was defined as a laparotomy created for any purpose other than specimen extraction. Patients who required conversion to open surgery (CONV) were matched 1:2 with laparoscopically completed cases (LAP) and 1:5 with patients undergoing open colorectal resection (OPEN). The matching criteria included age, American Society of Anesthesiologists (ASA) score, year of surgery (±3 years), tumor location (left, right, or rectum), and tumor node metastasis (TNM)-based tumor staging. Right colon location included cecum, ascending colon, hepatic flexure, and transverse colon, whereas the definition of left colon designated primaries originating from the splenic flexure, descending colon, sigmoid colon, or rectosigmoid junction. The definition of rectum was based on the confluence of the teniae. In addition, all cases of rectal carcinoma included in this study had a tumor distance of 15 cm or less from the anal verge.
The collected patient comorbidities included hypertension, coagulopathy, and diabetes, respiratory, renal, hepatic, and cardiovascular disease. The 30-day postoperative complications included bowel obstruction (diagnosed at operation or when the patient had at least 3 of the following 5 symptoms: nausea, abdominal pain, vomiting, abdominal distention, or absence of flatus or stools in the preceding 24 hours; and when plain X-ray or contrast studies favored bowel obstruction), anastomotic leak (based on an endoscopic finding or demonstration of contrast leak or on sinus originating from the anastomosis shown on imaging studies including gastrografin enema and CT scan), abdominal or pelvic abscess, peritonitis, deep venous thrombosis, pulmonary embolism, wound infection, and urinary, cardiovascular, or other respiratory morbidity.
The operative techniques of laparoscopic colectomy and rectal resection have been described previously [13, 14]. Both laparoscopic and open colorectal resections were performed according to the oncologic surgical principles of lymphovascular ligation at the origin of the main vessels and tailored mesorectal excision according to the distance of the rectal tumors from the anal verge.
Anastomotic techniques and use of proximal diverting ileostomy depended on the type of resection and the individual surgeon’s discretion. An R0 resection was defined as a resection with a distal margin clearance greater than 1 cm and a radial margin clearance exceeding 2 mm.
Categorical variables were analyzed using chi-square or Fisher’s exact test as appropriate, and the Wilcoxon rank sum test was used for quantitative and ordinal variables. Comparisons of overall and cancer-specific survival rates and comparisons of local and distant recurrence rates were performed using the Kaplan–Meier method. A P value less than 0.05 was considered significant. Computer matching and statistical analyses were performed using R version 2.4.1 (R foundation for statistical computing, Vienna, Austria).
Results
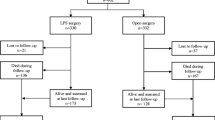
During the study period, 450 laparoscopic colorectal resections for cancer were evaluated, 45 (10%) of which required conversion to open surgery. After eliminations via the exclusion criteria (11 metastatic disease cases, 2 urgent surgery cases, and 1 palliative surgery case), 31 patients with updated oncologic follow-up data were included in the CONV group and compared with 62 matched LAP and 155 matched OPEN patients. The reasons for conversion included presence of a bulky tumor (12 cases, 38.7%), adhesions (11 cases, 35.5%), otherwise unclear anatomy (6 cases, 19.3%), and hemorrhage (2 cases, 6.5%).
Age, ASA score, surgery period, tumor location, and pathologic stage were similar between CONV and each control group as expected because of the case-matched study design. The incidence of overall comorbidities was not significantly higher in the CONV group (61.3%) than in the LAP (51.6%; P = 0.4) and OPEN (48.4%; P = 0.2) groups. An increased proportion of patients who required conversion were males (77.4%) compared with the LAP (59.7%) and OPEN (58.1%) groups, but the difference was borderline or not statistically significant (P = 0.09 and 0.05, respectively). Body mass index (BMI) in the CONV group was significantly higher than in the LAP group (29.6 vs 26.8; P = 0.012), whereas it was similar to that in the OPEN group (28.8; P = 0.3).
The rate of diverting loop ileostomy was similar among the groups. The intraoperative complication rate was significantly higher in CONV group (16.1% vs 4.8% in the LAP group; P = 0.1, and 16.1% vs 3.2% in the OPEN group; P = 0.02). The incidence of perioperative blood transfusion was higher than in the LAP group, although the difference was not significant (16.1% vs 6.5%; P = 0.15), and similar to that in the OPEN group (16.1% vs 12.9%; P = 0.6).
Overall morbidity and mortality at 30 days were comparable among the groups (Table 1). The CONV group and both control groups also were comparable in terms of tumor differentiation, median number of harvested lymph nodes, and incidence of positive resection margins. The rates of adjuvant chemotherapy and neoadjuvant chemoradiation were also similar (Table 2).
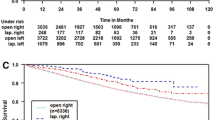
Long-term oncologic results are shown in Table 3. The median follow-up period for the CONV cases (4.1 years) was similar to that for the LAP (4.2 years, P = 0.9) and OPEN (4.6 years, P = 0.3) cases. Both the overall and disease-free survival rates were significantly lower in the CONV group than in the control groups. However, the corresponding cancer-related outcomes were similar among the groups.
Discussion
After a median follow-up period longer than 4 years, the cancer-specific mortality rates and the overall and local recurrence rates were similar among the CONV, LAP, and OPEN groups. Despite this, overall survival and disease-free survival were significantly decreased in the CONV group. Because death from any cause is included in the definition of disease-free survival [15], these results reflect an increased mortality unrelated to cancer in the CONV group.
The design of this study was planned considering that a comparison of outcomes between converted and laparoscopically completed patients or patients undergoing open procedure is subject to a significant discrepancy in patient case mix. In fact, conversion has been associated with several demographic and perioperative factors such as gender, comorbidities, tumor size and location, patient selection for a laparoscopic approach, intraoperative complications, and surgeon experience [11, 13, 14]. Because the unpredictable nature of conversion renders a randomized trial prohibitive, we decided to design our analysis as a case-matched study to minimize the influence of confounding variables.
Unlike our study, a number of series have suggested an adverse effect of conversion to open surgery on oncologic outcomes after laparoscopic colorectal resection for cancer. In particular, at least two studies indicated an increase in the local recurrence rate when conversion to open surgery was necessary [5, 8]. In these studies, both converted and laparoscopically completed procedures were retrospectively compared without any case matching. As a result, the conversion group had a greater proportion of advanced T-stage and rectal tumors than tumors at other locations and was associated with a higher rate of overall morbidity. An additional prospective series of laparoscopic proctectomies also suggested a trend in the association between conversion and a higher overall recurrence rate [10]. The authors reported a higher rate of stage 4 tumors in the converted group, which were excluded from the survival analysis. However, an increased number of patients with ASA 3 scores and N1 tumors still was associated with conversion, although this association was not statistically significant.
Our results also indicate that the CONV group was characterized by an increased rate of intraoperative complications. This could be a cause of conversion per se, as reported in other experiences [10]. Theoretically, a larger, more aggressive tumor could render the dissection difficult and thus be associated with both higher conversion rates and worsened oncologic outcomes. Our data do not support this hypothesis, which might depend on individual or institutional criteria of patient selection.
In spite of the case-matched design, our study had a number of limitations, particularly with regard to sample size and patient characteristics. First, although the sample size of our study population was comparable with that of similar series in the literature, it might still have been insufficient to indicate a significant association between conversion and worsened cancer outcomes. Future larger studies are therefore necessary to eliminate the possibility of a type 2 error in this regard.
Second, there was a greater percentage of male patients among the 31 CONV study patients than in the control groups. Male gender was not surprisingly associated with conversion during rectal cancer resection, probably due to the increased difficulty of rectal dissection in the narrower male pelvis. In fact, 9 out of 10 laparoscopic rectal resections in the CONV group were performed for male patients. Similarly, the mean BMI was significantly higher in the CONV group than in the LAP group. However, recent series have not indicated any correlation between either BMI or gender and oncologic outcomes [16, 17]. We therefore do not believe that this discrepancy significantly affected our analysis of oncologic outcomes.
Our CONV group was also characterized by more than an 80% rate of ASA 3 classification, which reflects overall comorbidity rates and is consistent with other data indicating an association between a higher ASA score and conversion [10, 11, 18]. However, the control groups also had an equivalent percentage of ASA 3 because ASA classification was one of the matching criteria used in our study. It is therefore possible that ASA classification could be inaccurate in evaluating the influence of comorbidities on long-term outcomes [19]. In particular, different types and degrees of comorbidity may have been included within the same ASA classification, which may have disproportionately affected the mortality rate in the CONV group. In fact, despite the ASA score matching among the groups, the overall comorbidity rate was higher in the CONV group than in the LAP and OPEN groups, albeit this difference was not statistically significant.
Tumor location was one of the matching criteria used in this study due to the different patterns of recurrence between colon and rectal cancers. However, it was not possible to perform separate analyses of colon and rectal resections due to the low number of CONV cases, which therefore had to be considered together in a combined group. With this limitation in mind, local recurrence rates were not increased in our cases of rectal cancer. In fact, only one case of local recurrence in the CONV group was observed in a rectal cancer location.
In conclusion, this case-matched study was specifically designed to reduce possible biases related to comorbidity, age, period of surgery, tumor stage, and location. In spite of a relatively small patient population, our results suggest that conversion to open surgery during laparoscopic colorectal resection for cancer does not affect oncologic survival or tumor recurrence. Results from larger, multicenter trials are necessary to confirm our conclusions.
References
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term end points of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Gervaz P, Pikarsky A, Utech M, Secic M, Efron J, Belin B, Jain A, Wexner S (2001) Converted laparoscopic colorectal surgery. Surg Endosc 15:827–832
Chan AC, Poon JT, Fan JK, Lo SH, Law WL (2008) Impact of conversion on the long-term outcome in laparoscopic resection of colorectal cancer. Surg Endosc 22:2625–2630
Moloo H, Mamazza J, Poulin EC, Burpee SE, Bendavid Y, Klein L, Gregoire R, Schlachta CM (2004) Laparoscopic resections for colorectal cancer: does conversion affect survival? Surg Endosc 18:732–735
Casillas S, Delaney CP, Senagore AJ, Brady K, Fazio VW (2004) Does conversion of a laparoscopic colectomy adversely affect patient outcome? Dis Colon Rectum 47:1680–1685
Ptok H, Steinert R, Meyer F, Kröll KP, Scheele C, Köckerling F, Gastinger I, Lippert H (2006) Long-term oncological results after laparoscopic, converted, and primary open procedures for rectal carcinoma: results of a multicenter observational study. Chirurg 77:709–717
Agha A, Fürst A, Iesalnieks I, Fichtner-Feigl S, Ghali N, Krenz D, Anthuber M, Jauch KW, Piso P, Schlitt HJ (2008) Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal Dis 23:409–417
Rottoli M, Bona S, Rosati R, Elmore U, Bianchi PP, Spinelli A, Bartolucci C, Montorsi M (2009) Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 16:1279–1286
Tekkis PP, Senagore AJ, Delaney CP (2005) Conversion rates in laparoscopic colorectal surgery: a predictive model with 1,253 patients. Surg Endosc 19:47–54
Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C, Gastrointestinal Cancer Disease-Site Group of Cancer Care, Ontario’s Program in Evidence-Based Care (2003) Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 6:3–26
Senagore AJ, Delaney CP, Brady KM, Fazio VW (2004) Standardized approach to laparoscopic right colectomy: outcomes in 70 consecutive cases. J Am Coll Surg 199:675–679
da Luz Moreira A, Mor I, Geisler DP, Remzi FH, Kiran RP (2011) Laparoscopic resection for rectal cancer: a case-matched study. Surg Endosc 25:278–283
Chua YJ, Sargent D, Cunningham D (2005) Definition of disease-free survival: this is my truth-show me yours. Ann Oncol 16:1719–1721
Ballian N, Yamane B, Leverson G, Harms B, Heise CP, Foley EF, Kennedy GD (2010) Body mass index does not affect postoperative morbidity and oncologic outcomes of total mesorectal excision for rectal adenocarcinoma. Ann Surg Oncol 17:1606–1613
Takahashi T, Kato T, Kodaira S, Koyama Y, Sakabe T, Tominaga T, Hamano K, Yasutomi M, Ogawa N (1996) Prognostic factors of colorectal cancer: results of multivariate analysis of curative resection cases with or without adjuvant chemotherapy. Am J Clin Oncol 19:408–415
Tan PY, Stephens JH, Rieger NA, Hewett PJ (2008) Laparoscopically assisted colectomy: a study of risk factors and predictors of open conversion. Surg Endosc 22:1708–1714
Chandra A, Mangam S, Marzouk D (2009) A review of risk scoring systems utilised in patients undergoing gastrointestinal surgery. J Gastrointest Surg 13:1529–1538
Disclosures
Matteo Rottoli, Luca Stocchi, Dan P. Geisler, and Ravi P. Kiran have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rottoli, M., Stocchi, L., Geisler, D.P. et al. Laparoscopic colorectal resection for cancer: effects of conversion on long-term oncologic outcomes. Surg Endosc 26, 1971–1976 (2012). https://doi.org/10.1007/s00464-011-2137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-2137-8