Abstract
Background
Among the possible complications of bariatric surgery, fistula and partial dehiscence of the gastric suture are well known. Reoperation often is required but results in significant morbidity. Endoscopic treatment of some bariatric complications is feasible and efficient.
Methods
A modified metallic stent was placed between the gastroaesophageal junction and the alimentary jejunal limb, allowing the passage of a nasoenteric feeding tube into the jejunal limb.
Results
Endoscopy showed disruption of nearly the entire staple line at the gastric pouch. The modified stent was placed and allowed wound healing. After 31 days, the stent had migrated and was removed endoscopically. Total clousure of the fistula was reported 30 days afterward.
Conclusions
Endoscopic treatment of some bariatric surgery complications is feasible and has been reported previously. This report presents a case of a serious leakage treated by placement of a self-expandable metal stent to bridge the fistula.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is an invasive procedure associated with few complications in expert hands, but fistula and dehiscence may occur [1–3]. Reoperation often is required but results in significant morbidity.
The diagnosis and treatment of bariatric surgery leakage are challenging due to nonspecific clinical examination and laboratory findings. Some cases are associated with situations of extreme gravity, narrowing the options of treatment and thus making the complications very difficult to solve [3–6].
We present an unusual case involving a large fistula of the gastric pouch due to extensive dehiscence of the staple line leading a critical clinical condition of the patient. Endoscopic treatment was used to manage this case.
Materials and methods
A 32 year-old woman submitted to an Roux-en-Y gastric bypass (RYGB) showed signs of sepsis due to leak on postoperative day 7. To control the leak site, the patient was submitted to two reoperations, but because sepsis continued, another surgery was performed for drainage, ending in laparostomy. At this time, the patient was transferred to the author’s service, and a surgical exploration showed leaks from three sites in the upper abdomen, namely, the disrupted staple line from both the gastric pouch, the remnant stomach, and the gastrojejunal anastomosis (Fig. 1).
The drainage tubes were exchanged, and a vacuum dressing was used to control the sepsis and drive the leaks. Because the surgical team believed that access to the gastrojejunostomy using conventional techniques was not feasible and because both computed tomography (CT) scan and die tests were not helpful, an upper endoscopy was performed as the next diagnostic test.
Results
The examination showed that almost the entire staple line at the gastric pouch and most of the gastrojejunostomy were disrupted. It was possible to access to the abdominal cavity with the endoscope and identify disruption at the upper part of the remnant stomach. The jejunal alimentary limb of the gastrojejunostomy also could be accessed by the endoscope (Figs. 2, 3).
The case was discussed with a multidisciplinary team including the surgery staff, interventional radiology, and endoscopy. The option of a new surgical procedure under these circunstances was considered unsafe, but an alternative procedure was needed because the patient remained in serious condition, and the vacuum dressing could not stay indefinitely.
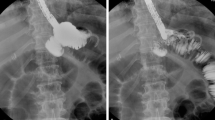
The choice was to “reconnect” the gastric pouch to its gastrojejunostomy with a stent and to allow wound healing by a second intention. A fully covered self-expandable metal stent (SEMS) was modified for better adaptation to the surgically modified anatomy after RYGB. This modified SEMS had a smaller diameter (27 mm) at its proximal portion located above the gastroesophageal junction and a larger diameter (30 mm) at the distal portion located on the pouch and the gastrojejunal anastomosis. The authors used this modified stent delivered over a Savary bougie guidewire under radiologic guidance so that the distal esophagus was connected with the jejunal alimentary limb. A nasoenteric tube was left on the alimentary limb to improve the patient’s nutritional status. The disrupted remnant stomach was left to heal by second intention with the vacuum dressing (Figs. 4, 5).
After stenting, the patient’s clinical status improved dramatically as well as the amount of leak (decreasing from 1,300 to 100 ml daily withinin 5 days). With improvement in the patient’s clinical status, she was discharged from intensive care unit (ICU) and referred to home care.
The patient was readmitted on an emergency basis 31 days after stenting with abdominal pain. An abdominal X-ray showed that the stent had dislodged into the bowel. Upper endoscopy was performed using fluoroscopic guidance with the patient under general anesthesia. The stent was removed without complications using a double-channel scope. The previous disrupted staple lines seemed to be healed. Nevertheless, it was decided to place a nasoenteric feeding tube on the alimentary limb. The patient had an uneventful clinical outcome, with complete clousure of the laparostomy. Upper endoscopy performed 60 days after stent placement showed a standard RYGB endoscopic anatomy with no signs of leaks or fistula (Fig. 6).
Discussion
Obesity is recognized as a multifactorial disease with involvement of genetic, endocrine, metabolic, social, psychological, and comportmental factors in its origin. Over the past decade, the prevalence of obesity has increased, becoming a public health problem that affects more than 33% of adults in the United States. Direct obesity-related health spending costs are estimated to be $147 billion annually, double what they were a decade ago and amounting to nearly 10% of medical spending [7–9].
Surgery has proved to be the most effective long-term weight loss therapy, reducing complications and improving the quality of life for a large number of morbidly obese patients. Gastric bypass and sleeve gastrectomy are the techniques performed most often, both with significant weight loss and low complication rates in experienced hands.
A large study of 38,501 patients submitted to bariatric surgery reported a 30-day mortality rate of 0.24%, with deaths most often due to pulmonary embolism, major cardiac events, and gastrointestinal (GI) leak, respectively. The major complication rate was 3.4% (GI leak, 0.7%; bleeding, 0.44%; small bowel obstruction, 0.4%) [10]. Male patients older than 55 years with multiple comorbid conditions, especially diabetes and hypertension, undergoing revisional surgery are at increased risk for anastomotic leak and death [9, 11].
Except for pulmonary embolism, anastomotic leak is the leading cause of death after bariatric surgery, and patients with a symptomatic leak requiring reintervention have a mortality rate of 10 to 16% [11, 12]. The diagnosis often is challenging, requiring a high level of suspicion and quick evaluation with diagnostic exams because the patients often show few or no clinical symptoms other than changes in the drainage tube fluid, and clinical examination is limited due to patient weight.
Treatment depends on several factors, mainly clinical symptoms, systemic signs of infection, and effectiveness of drainage. Patients who are septic or symptomatic and those who have inadequate drainage are better treated with a surgical procedure. The most important measure of treatment for these patients is to control the source of the sepsis through adequate drainage, support with broad-spectrum antibiotics, and if available, close vigilance under ICU [6, 10, 13, 15, 16].
Most asymptomatic gastric leaks in stable patients can be submitted to conservative treatment, and endoscopy has proved to be a safe and effective way to provide enteral nutrition through enteral feeding tubes or stents. However, attempts to close the fistula site with clipping devices, synthetic glues, or mesh plugs have shown controversial results.
Studies have shown that the use of stents for selected patients with bariatric anastomotic leaks supports healing, provides enteral nutrition, avoids morbid procedures, and shortens the hospital stay [12]. It is an ideal treatment because it is effective, less morbid than surgery, and not affected by the patient’s weight (an independent risk factor for leak). But it nevertheless results in some complications, primarily migration, bleeding, and intolerance [13–15].
A recent study investigating the long-term outcome of stent therapy for bariatric surgery complications analyzed 26 patients with leaks, strictures, or fistula who underwent stent therapy. The rate for resolution of the initial complication was 85%, but the findings showed a stent migration rate of 40%. Of these migrations, 82% could be removed endoscopically, 9% passed through the entire GI tract, and 4% required laparoscopic stent removal. In this study, both polyester and nitinol stents were used, but as the study progressed, more nitinol stents were deployed due to lower migrations rates. Use of longer and overlapping stents reduced migration rates to 27% [12].
Different strategies adopted to reduce migration rates such as the use of long and overlapping stents and partially covered stents with retraction mechanisms are valid and should be pursued. In this study, we report a modification of the stent design that allowed control of a severe leak due to staple line dehiscence in a septic patient with no surgical access to the leak site, illustrating a less aggressive and yet interesting alternative to endoscopic treatment of an extremely dificult bariatric complication [14].
In conclusion, several publications have reported successful endoscopic treatment of bariatric complications such as strictures or fistulas. Anastomotic leak, one of the most severe bariatric complications with a high morbidity rate, can be treated successfully by a feasible, effective, and life-saving endoscopic treatment.
References
Hahler B, Schassberger D, Novakovic R, Lang S (2009) Managing complex, high-output, enterocutaneous fistulas: a case study. Ostomy Wound Manage 55:30–42
Martin-Malagon A, Arteaga-Gonzalez I, Rodriguez-Ballester L, Diaz-Romero F (2010) Gastroesophageal junction leak with serious sepsis after gastric bypass: successful treatment with endoscopy-assisted intraluminal esophageal drainage and self-expandable covered metal stent. Obes Surg 20:240–243
Thaler K (2009) Treatment of leaks and other bariatric complications with endoluminal stents. J Gastrointest Surg 13:1567–1569
Yurcisin BM, DeMaria EJ (2009) Management of leak in the bariatric gastric bypass patient: reoperate, drain, and feed distally. J Gastrointest Surg 13:1564–1566
Edwards CA, Bui TP, Astudillo JA, de la Torre RA, Miedema BW, Ramaswamy A, Fearing NM, Ramshaw BJ, Thaler K, Scott JS (2008) Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis 4:594–599
Campos JM, Moura EGH (2008) Fïstula gastrojejunal in endoscopia em cirurgia da obesidade, vol 1, 1st edn. Livraria Santos Editora Ltda, São Paulo-SP, p 435
Flegal KM (2010) Prevalence and trends in obesity among U.S. adults, 1999–2008. J Am Med Assoc 303:235–241
Finkelstein EA et al (2009) Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff 29:w822–w831
Sturm R (2007) Increases in morbid obesity in the USA: 2000–2005. Public Health 121:492–496
Fernandez AZ Jr, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ (2004) Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc 18:193–197 Epub 29 December 2003
Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M (2006) Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg 192:657–662
Mejía AF, Bolaños E, Chaux CF, Unigarro I (2007) Endoscopic treatment of gastrocutaneous fistula following gastric bypass for obesity. Obes Surg 17:544–546
Ballesta C, Berindoague R, Cabrera M, Palau M, Gonzales M (2008) Management of anastomotic leaks after laparoscopic Roux-en-Y gastric bypass. Obes Surg 18:623–630 Epub 8 April 2008
Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M (2006) Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg 192:657–662
Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC, Barthet M (2011) An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 73:238–244
Campos JM, Moura EGH (2008) Tratamento endoscópico de fístula com prótese auto-expansível in endoscopia em cirurgia da obesidade. Vol 1, 1st edn. Livraria Santos Editora Ltda, São Paulo-SP, p 435
Disclosures
Eduardo G. H. de Moura, Manoel P. Galvão-Neto, Almino C. Ramos, Eduardo T. H. de Moura, Thales D. Galvão, Diogo T. H. de Moura, and Flávio C. Ferreira have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 56,354 kb)
Rights and permissions
About this article
Cite this article
de Moura, E.G.H., Galvão-Neto, M.P., Ramos, A.C. et al. Extreme bariatric endoscopy: stenting to reconnect the pouch to the gastrojejunostomy after a Roux-en-Y gastric bypass. Surg Endosc 26, 1481–1484 (2012). https://doi.org/10.1007/s00464-011-2060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-2060-z