Abstract
Both in endoscopic bariatric therapy and in post-bariatric surgical complications where the endoscopist is consulted and called up for help, the endoscopist may request earnestly the assistance of a surgeon, a true cross-pollination between two specialisms and two specialists.
Examples in endoscopic bariatric therapy are cases of perforation during balloon insertion or caused by the balloon itself during its residence in the stomach. The duodenojejunal bypass sleeve has certain complications such as device migration, gastrointestinal bleeding and liver abscess during their stay and complications due to mechanical trauma upon removal such as oesophageal perforation. For some endoscopic bariatric therapies, the endoscopist has to rely on laparoscopic assistance such as with ValenTx oesophagogastroduodenal bypass sleeve and with the incisionless magnetic anastomotic system to create a jejunoileal bypass. Eventually, we should consider the possibility of “third-stage complication”, i.e. first a surgical complication that is treated by the endoscopist, which may then be followed by a potential complication of the endoscopy that should be treated by surgery. Examples thereof are stent migration, perforation after endoscopic dilation of a surgical stenosis, an intractable stenosis after many endoscopic dilations that need surgery again, secondary abscesses after endoscopic drainage that cannot be approached by radiology and a persistent fistula despite all endoscopic measures.
In post-bariatric surgical complications the endoscopist, on his/her turn, needs the assistance of the surgeon, as for instance in the access to the excluded stomach and the biliary tree. Then two teams of surgeons and endoscopists are needed for the laparoscopy-assisted ERCP (LA-ERCP) and the laparoscopic transgastric rendez-vous (LATG-RV) procedure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Obesity
- Overweight
- Endoscopic bariatric therapy
- Intragastric balloon
- Duodenojejunal bypass sleeve
- Gastric suturing
- Gastric plication
- ValenTx oesophagogastroduodenal bypass sleeve
- Incisionless magnetic anastomotic system
- Laparoscopy-assisted ERCP
- Laparoscopic transgastric rendez-vous procedure
Both in endoscopic bariatric therapy and in post-bariatric complications where the endoscopist is consulted and called up for help, the endoscopist may request earnestly the assistance of a surgeon, a true cross-pollination between two specialisms and specialists.
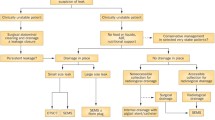
Although intragastric balloons date back to the 1980s, endoscopic bariatric therapy is a relatively new sprout on the tree of knowledge of how to treat obesity and lags far behind the surgical experience in this field. Endoscopic techniques to treat obesity stand in between medical treatment and surgical interventions and, as long as they have not proven to serve as an independent, efficacious and long-standing approach, they should always consider – in their aggressiveness on the stomach – the feasibility of bariatric surgery in the future. Modern endoscopic bariatric operations are in a constant state of development, evolution and technical upgrading. This will create several kinds of post-endoscopic issues that should be handled in combination with bariatric surgeons. In the first place, there are immediate or early but also later, more postponed complications that may be managed conservatively or need the help of a surgeon. Even when conservative management is considered, it is always wise to inform the surgeon. Examples where the endoscopist needs the assistance of a surgeon are perforations of the stomach either during placement of an intragastric balloon or later, when the balloon itself causes a gastric perforation. Surgery is then required and to reduce the duration of the operation and the injury to the gastric wall surgeons in their turn often ask endoscopists to remove the balloon by endoscopy in the same setting [1]. Abou Hussein et al. described this approach in 3 cases and reviewed the literature where in total 18 cases were found with 3 deaths [1]. Such a high mortality rate raises doubts about recommendations by Bekheit et al., who managed a gastric perforation secondary to intragastric balloon insertion successfully by conservative means and concluded that such perforations can be treated conservatively in highly selected patients [2]. The balloon can be removed through the perforation itself if large enough by the surgeon. An oesophageal perforation arising during balloon removal may be treated conservatively, depending on the size of the tear, either by a suction tube at the level of the tear, antibiotics and nil per mouth or by placing a stent [3, 4]. This perforation is different from a perforation occurring during dilation of a stenosis. While patients in both cases are fasting, the balloon is often covered with unmeshed food, which may enter and contaminate the area of perforation. So, in case of stent placement one should be prepared for the development of an abscess. When a balloon deflates and cannot be retrieved from the stomach by the endoscopist, the patient should be observed for clinical signs of small-bowel obstruction, and the endoscopist should be deliberate with the surgeon if and when surgical intervention is needed. Sometimes, transabdominal puncture to deflate the balloon by ultrasound is possible as was possible in two of the three balloons that obstructed the small intestine, thus bypassing the need of surgery [5]. Bleeding upon balloon placement or balloon removal may be due to a Mallory-Weiss lesion and is usually treated conservatively or by endoscopic clipping [3, 5]. However, bleeding from an ulcer induced by pressure necrosis or by damage to the wall may be more difficult as the balloon has to be removed first, followed by endoscopic haemostasis techniques [4, 5]. Because of a change in the design of the ReShape Duo intragastric balloon, the ulcer rate went down from 39.6 to 10.3%, and only one of the initial ulcers that were also larger in size bled and needed intervention [4]. In the pivotal US study with the swallowable Obalon balloon only one haemorrhage occurred that was treated conservatively [6]. In the presence of a fulminant bleeding that cannot be controlled by endoscopic measures, there are two choices: that of radiologic embolisation or surgical intervention.
The duodenojejunal bypass sleeve (DJBS) has certain complications such as device migration (4.9%), gastrointestinal bleeding (3.9%) and liver abscess (0.13%) during their stay and complications due to mechanical trauma upon removal such as oesophageal perforation (0.13%) [7, 8]. As is the case with balloon removal, the possibility of a mucosal tear and oesophageal perforation exists by the sharp barbs that hold the DJBS in place and that should be encapsulated within the protective plastic foreign-body retrieval hood mounted on the endoscope to avoid trauma to the stomach or oesophagus. An uncovered barb caused an oesophageal perforation upon withdrawal of the sleeve [7]. A very exceptional case among 21 DJBS cases has been described [9]. This patient suffered from an acute cholecystitis and duodenal fistula due to bulbar transmural penetration and gallbladder impaction by one of the anchors/barbs of the DJBS 1 month after the implant [9]. DJBS migration can mostly be treated by endoscopy but also once a surgical intervention was needed. Gastrointestinal bleeding can be a major complication when the DJBS corrodes the posterior duodenal artery, which can be so massive to not allowing to remove the sleeve and to re-endoscope the patient. Moreover, the positioning at the rear of the duodenal bulb may thwart a favourable approach for endoscopic haemostasis. In these cases radiologic embolisation is the preferred method and surgery is the second choice. Fortunately, however, the eight bleedings reported by Tarnoff et al. and Gershin et al., although impressive by their demonstration of haematemesis, occurred at the proximal anchor point in the oesophagus and did not need major interventions such as embolisation or surgery [10, 11]. Liver abscesses, reported in only 0.13% in Abu Dayyeh’s analysis, but being the main reason of interruption of the US pivotal study because of their occurrence in 3.5%, seldom need a surgical approach as these can be drained by the radiologist [7, 12].
All these complications can be treated by a general of a gastrointestinal surgeon, who should, however, be experienced with laparoscopy and should have knowledge of the special obesity-related problems of less visibility due to the large liver and a fatty and large mesentery, and less manoeuvrability of instruments due to the thick abdominal wall (all of this being nowadays part of the basic training in laparoscopic digestive surgery). This may be different for the more invasive techniques such as gastric suturing or stapling where a bariatric surgeon might be preferred. Both in the Primary Obesity Surgery Endoluminal (POSE) with the Incisionless Operating Platform and in the OverStitch studies intraluminal and extraluminal bleedings have been reported. The intraluminal bleedings emanated from the stitches and sutures and could be treated by the endoscopist. Intraluminal bleeding occurred in 2 of the 20 patients in Lopez-Nava’s study using the OverStitch [13]. Two endoscopically treated bleedings in 34 patients and minor bleedings in 147 patients of whom 1 needed hospitalisation were found in two POSE studies [14, 15]. In a multicentre survey of the OverStitch one splenic laceration with a bleeding (0.4%) occurred and in the US pivotal study, the ESSENTIAL study concerning the POSE, one extraluminal gastric bleeding (0.4%) was seen which needed surgical intervention [16, 17]. True symptomatic gastric perforations both after suturing and after gastric plication always needed surgery, whereas pneumoperitoneum was asymptomatic and left untreated or managed conservatively [18, 19]; a case of pneumothorax and pneumoperitoneum needed a chest tube [16]. Two perigastric fluid collections adjacent to the fundus were drained locally [16]. A rare case of incarceration of the gallbladder with endoscopic stitches has been reported that was explained by the lateral position of the patient during the OverStitch procedure.
From these data it is obvious that although conservative treatment may suffice in many cases, the trust in readily accessible surgical help is mandatory. For some endoscopic bariatric therapies, the endoscopist relies on laparoscopic assistance, but this may change in the near future. To avoid a faulty suturing for fixation of the upper part of the ValenTx oesophagogastroduodenal bypass sleeve and to avoid interjacent bowel loops between the two magnets of the incisionless magnetic anastomotic system (IMAS) to create a jejunoileal bypass, these endoscopic procedures are performed under laparoscopic control [20, 21].
As mentioned earlier, the endoscopist should realise that sutures, plications or staples but also sequelae from the duodenal bypass sleeve or intragastric balloons may hamper a bariatric procedure later in life. So, it is important that in their follow-up reports they report which patients needed bariatric surgery in a later stage and to what extent surgeons were troubled by the endoscopic procedure. Presumed or observed thickening of the gastric wall after intragastric balloon treatment is a reason for some surgeons to postpone bariatric surgery for 2 weeks [22]. However, others have not seen any problems and many reports are available of removal of the balloon and bariatric surgery in the same session [23, 24]. Notwithstanding the major intra-abdominal changes observed at the outside of the stomach in animal studies after DJBS, those changes were not seen in the study by Gershin et al. where patients, after a period of DJBS treatment, underwent LAGB and RYGB uneventfully [11]. Yet, three studies reported local inflammation and pseudopolyp formation at the inside, during device removal and up to 2–4 weeks after device removal [10, 25, 26]. Also, gastric plication after a Transoral Endoscopic Restrictive Implant System (TERIS) did not interfere with subsequent RYGB surgery. Much less experience with subsequent surgery is available for the POSE and OverStitch procedures. Besides the materials that have been left in place and often cannot be removed, as they are deep rooted in the tissues, also adhesions as a result of the endoscopic bariatric surgery or resulting from a complication may hinder the bariatric surgeon. Perigastric collections near the fundus may be such an example.
The complication in itself but also the consequences of previous endoscopic bariatric therapy as discussed before should be discussed by both endoscopist and surgeon, not only to decide what to do but also to see to what extent these changes are a technical barrier for the surgeon, such as stapling within or across plicated folds and thicker tissue, but also to discuss sequential strategies that might follow after this complicated endoscopic bariatric treatment. The specifics of surgery become different: for example, during a sleeve gastrectomy, the stapler cartridges might run through the plicated stomach with potential anchors situated on the greater curve, while when performing a bypass the lesser curve should be preserved. Under these circumstances, careful pre-surgical endoscopy is the key element to a roadmap for the surgeon. Moreover, in the discussion of a surgical procedure one should evaluate the response to the preceding endoscopic procedure, whereby algorithms that have been suggested by Ajuha and Nimgaonkar might be useful [27].
It is not possible to foresee all kind of complications that may occur during a given endoscopic procedure and that should be treated by a general of gastrointestinal surgeon. Albeit bariatric knowledge is recommended when dealing with such complications, surgeons with a general surgery background may often be in charge when such complications occur, which, to be fair, is nowadays occasionally also the case with post-bariatric surgery complications. This is certainly not the ideal situation but is not a particular threat when dealing with basic complications such as bleeding and sepsis with or without perforation.
Eventually, we should consider the possibility of “third-stage complication,” i.e. first a surgical complication that is treated by the endoscopist, which may then be followed by a potential complication of the endoscopy that should be treated by surgery and/or sometimes by radiology with a surgical backup. Examples thereof are stent migration, perforation after endoscopic dilation of a surgical stenosis, an intractable stenosis after many endoscopic dilations that need surgery again for seromyotomy or a revision, secondary abscesses after endoscopic drainage that cannot be approached by radiology, a persistent fistula despite all endoscopic measures, etc. Moreover, as has been discussed extensively in Chaps. 5 and 6, surgeons asked the help of an endoscopist in certain complications, who for being successful, on his/her turn, needs the assistance of the surgeon, as for instance in the access to the excluded stomach and the biliary tree. These two teams of surgeons and endoscopists are needed for the laparoscopy-assisted ERCP (LA-ERCP) and the laparoscopic transgastric rendez-vous (LATG-RV) procedure, performed in cases where also a cholecystectomy is indicated [28,29,30]. Both success rate and costs have to be taken into account: Schreiner et al. demonstrated a higher success rate with the LA-ERCP when compared with balloon enteroscopy-assisted ERCP (BEA-ERCP), but cost-effectiveness calculations suggested to start with a BEA-ERCP [31]. However, when balloon enteroscopy is not available, the choice for a LA-ERCP is evident. The ERCP procedures through a gastrostomy, usually two-stage procedures, can also be performed as one-stage procedures when T-anchors for the apposition of gastric and abdominal walls are used. The two-stage approach involves first the creation of a gastrostomy and maintenance with a large-calibre catheter, followed by dilation after tract maturation which usually takes 4 weeks and ERCP via the gastrostomy tract. Successful access to the excluded stomach and creation of a gastrostomy have been previously described using various techniques, including a surgical gastrostomy [32, 33]. In contrast to other techniques such as radiology or gastrostomy, which need the maturation of the gastrostomy tract, a surgical gastrostomy allows an ERCP in the same session.
In conclusion, these examples emphasise the need for a true alliance and cooperation between endoscopists and surgeons. Future techniques, whether surgical or endoscopic, as well as unforeseen complications of the existing ones, make this reality even more obvious.
Abbreviations
- BEA-ERCP:
-
Balloon enteroscopy-assisted ERCP
- DJBS:
-
Duodenojejunal bypass sleeve
- ERCP:
-
Endoscopic retrograde cholangiopancreaticography
- IMAS:
-
Incisionless magnetic anastomotic system
- IOP:
-
Incisionless Operating Platform
- LA-ERCP:
-
Laparoscopy-assisted ERCP
- LAGB:
-
Laparoscopic adjustable gastric banding
- LATG-RV:
-
Laparoscopic assisted transgastric rendez-vous
- POSE:
-
Primary Obesity Surgery Endoluminal
- RYGB:
-
Roux-en-Y gastric bypass
- SG:
-
Sleeve gastrectomy
- TERIS:
-
Transoral endoscopic restrictive implant system
References
Abou Hussein BM, Khammas AA, Al Ani AM, Swaleh AH, Al Awadhi SA, El Tayyeb YH, et al. Gastric perforation following intragastric balloon insertion: combined endoscopic and laparoscopic approach for management: case series and review of literature. Obes Surg. 2016;26:1127–32.
Mohamed Bekheit M, Abdelsalam WN, Sgromo B, Catheline J-M, Katri K. Is conservative management for gastric perforation secondary to intragastric balloon possible? Case report and review of literature. Obes Surg. 2014;24:968–70.
Mathus-Vliegen EMH. Is endoscopy really necessary for placing intragastric balloons? Obes Surg. 2018;28(1):169–75. https://doi.org/10.1007/s11695-017-2812-5.
Ponce J, Woodman G, Swain J, Wilson E, English W, REDUCE Pivotal Trial Investigators, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874–81.
Mathus-Vliegen EMH, Tytgat GNJ. Intragastric balloons for morbid obesity: results, patient tolerance, and balloon life span. Br J Surg. 1990;77:76–9.
Sullivan S, Swain JM, Woodman G, Edmundowicz S, Hassanein TI, Shayani V, et al. 812d The Obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6-month randomized sham controlled trial. Gastroenterology. 2016;150:S1267.
ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee, Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425–38.
Brethauer SA, Chang J, GalvaoNeto M, Greve JW. Gastrointestinal devices for the treatment of type 2 diabetes. Surg Obes Relat Dis. 2016;12:1256–61.
Vilarrasa N, Ruiz de Gordejuela AG, Casajoana A, Duran X, Toro S, Espinet E, et al. Endobarrier® in grade I obese patients with long-standing type 2 diabetes: role of gastrointestinal hormones in glucose metabolism. Obes Surg. 2017;27:569–77.
Tarnoff M, Rodriguez L, Escalona A, Ramos A, Neto M, Alamo M, et al. Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc. 2009;23:650–6.
Gersin KS, Rothstein RI, Rosenthal RJ, Stefanidis D, Deal SE, Kuwada TS, et al. Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc. 2010;71:976–82.
Kaplan LM, Buse JB, Mullin C, Edmundo-Wicz S, Bass E, Visintainer P, et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral antihyperglycemic agents. Diabetes. 2016;65(suppl):A326–LB.
Lopez-Nava G, Galvão MP, da Bautista-Castaño I, Jimenez A, De Grado T, Fernandez-Corbelle JP. Endoscopic sleeve gastroplasty for the treatment of obesity. Endoscopy. 2015;47:449–52.
López-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The Primary Obesity Surgery Endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 2015;11:861–5.
Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after posesm vs. medical therapy. Obes Surg. 2017;27:310–22.
Lopez-Nava G, Sharaiha RZ, Galvao Neto M, Kumta NA, Topazian M, Shukla A, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 242 patients with 18 months follow-up. Gastroenterology. 2016;150:S26.
Sullivan S, Swain JM, Woodman G, Antonetti M, De La Cruz-Munoz N, Jonnalagadda SS, et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity. 2017;25:294–301.
Familiari P, Costamagna G, Blero D, Le Moine O, Perri V, Boskoski I, et al. Transoral gastroplasty for morbid obesity: a multicenter trial with a 1-year outcome. Gastrointest Endosc. 2011;74:1248–58.
de Jong K, Mathus-Vliegen EM, Veldhuyzen EA, Eshuis JH, Fockens P. Short-term safety and efficacy of the trans-oral endoscopic restrictive implant system for the treatment of obesity. Gastrointest Endosc. 2010;72:497–504.
Sandler BJ, Rumbaut R, Swain CP, Torres G, Morales L, Gonzales L, et al. One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg Endosc. 2015;29:3298–303.
Machytka E, Bužga M, Zonca P, Lautz DB, Ryou M, Simonson DC, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in subjects with obesity and diabetes. Gastrointest Endosc. 2017;86(5):904–12. https://doi.org/10.1016/j.gie.2017.07.009.
Jones M, Healey AJ, Efthimiou E. Early use of self-expanding metallic stents to relieve sleeve gastrectomy stenosis after intragastric balloon removal. Surg Obes Relat Dis. 2011;7:e16–7.
de Goederen-van der Meij S, Pierik RG, Oudkerk PM, Gouma DJ, Mathus-Vliegen LM. Six months of balloon treatment does not predict the success of gastric banding. Obes Surg. 2007;17:88–94.
Khan O, Irukulla S, Sanmugalingam N, Vasilikostas G, Reddy M, Wan A. Simultaneous intra-gastric balloon removal and laparoscopic sleeve gastrectomy for the super-super obese patients — a prospective feasibility study. Obes Surg. 2013;23:585–7.
Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55–9.
Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251:236–43.
Ahuja NK, Nimgaonkar A. Precision Bariatrics: toward a new paradigm of personalized devices in obesity therapeutics. Obes Surg. 2016;26:1642–5.
Badaoui A, Malherbe V, Rosiere A, De Ronde T. ERCP by laparoscopic transgastric access and cholecystectomy at the same time in a patient with gastric bypass who was seen with choledocholithiasis. Gastrointest Endosc. 2010;71:212–4.
Bertin PM, Singh K, Arregui ME. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography (ERCP) after gastric bypass: case series and a description of technique. Surg Endosc. 2011;25:2592–6.
Mejía R, Achurra P, Gabrielli M, Briceño E, Rebolledo R, Torres A, et al. Laparoscopy-assisted trans-gastric rendez-vous for the treatment of common bile duct stones in patients with prior Roux-en-Y gastric bypass. Obes Surg. 2016;26:2809–13.
Schreiner MA, Chang L, Gluck M, Irani S, Gan I, Brandabur JJ, et al. Laparoscopy–assisted versus balloon enteroscopy–assisted ERCP in bariatric post–Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–56.
Gutierrez JM, Lederer H, Krook JC, Kinney TP, Freeman ML, Jensen EH. Surgical gastrostomy for pancreatobiliary and duodenal access following Roux-en-Y gastric bypass. J Gastrointest Surg. 2009;13:2170–5.
Tekola B, Wan AY, Ramanath M, Burnette B, Ellen K, Schirmer BD, et al. Percutaneous gastrostomy tube placement to perform transgastrostomy endoscopic retrograde cholangiopancreaticography in patients with Roux-en-Y anatomy. Dig Dis Sci. 2011;56:3364–9.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mathus-Vliegen, E.M.H., Dargent, J. (2018). When the Endoscopist Needs the Surgeon. In: Bariatric Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-90074-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-90074-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90073-5
Online ISBN: 978-3-319-90074-2
eBook Packages: MedicineMedicine (R0)