Abstract
Background
Robotic colorectal surgery is gaining interest in general and colorectal surgery. The use of the da Vinci® Robotic system has been postulated to improve outcomes, primarily by increasing the dexterity and facility with which complex dissections can be performed. We report a large, single institution, comparative study of laparoscopic and robotic colectomies, attempting to better elucidate the benefits of robotic surgery in patients with colorectal disease.
Methods
We conducted a retrospective review of 171 patients who underwent robotic and laparoscopic colectomies (79 and 92, respectively) at our institution between November 2004 and November 2009. Patients in both groups had well-matched preoperative parameters. All cases were further subdivided by their anatomical location into right-sided and left-sided colectomy, and analysis was performed within these two subgroups. Perioperative outcomes reported include operative time, operative blood loss, time to return of bowel function, time to discontinuation of patient controlled analgesia, length of stay, and intraoperative or postoperative complications.
Results
Our results indicate that there is no statistical difference in length of stay, time to return of bowel function, and time to discontinuation of patient-controlled analgesia between robotic and laparoscopic left and right colectomies. Interestingly, the total procedure time difference between the laparoscopic and robotic colectomies was much smaller than previously published accounts (mean 140 min vs. 135 min for right colectomy; mean 168 min vs. 203 min for left colectomy).
Conclusions
Our study is one of the largest reviews of robotic colorectal surgery to date. We believe that our results further demonstrate the equivalence of robotic surgery to laparoscopic surgery in colorectal procedures. Future research should focus on surgeon-specific variables, such as comfort, ergonomics, distractibility, and ease of use, as other ways to potentially distinguish robotic from laparoscopic colorectal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Robotic colorectal surgery is gaining interest in general and colorectal surgery. The da Vinci® Robotic system (Intuitive Surgical Inc., Sunnyvale, CA), currently the only commercially available telerobotic technology on the market, has been postulated to improve outcomes over traditional open and laparoscopic surgery, primarily by increasing the dexterity and facility with which complex dissections are performed. Theoretical advantages for the surgeon include [1, 2]:
-
A stereoscopic, three-dimensional view allowing for complete immersion in the operative field
-
Special articulating instrumentation offering multiple additional degrees of freedom
-
Motion scaling permitting more precise dissection
-
Ergonomic operative position for increased comfort
Surprisingly, the theoretical promise of robotics has not yet fully penetrated the practice of general surgery. The current literature has not demonstrated any measurable difference for operative and postoperative outcomes between patients with benign or malignant colon disease who undergo laparoscopic and robotic procedures [3–5]. However, only a handful of published accounts involving robot-assisted colorectal surgery exist, composed mainly of case reports and series [6–8], and these are fraught with selection bias, small sample sizes, and pooled institutional data. We report a large, single institution, comparative study of laparoscopic and robotic colectomy, attempting to better elucidate the benefits of robotic surgery in patients with colorectal disease.
Materials and methods
We conducted a retrospective review of 171 patients who underwent robotic and laparoscopic colectomies (79 and 92, respectively) at our institution between November 2004 and November 2009. Experienced general surgeons, trained in minimally invasive techniques using laparoscopic and robotic technology, performed all procedures. The study was approved by the North Shore–Long Island Jewish Health System Institutional Review Board (IRB Protocol #09-160).
The two groups, laparoscopic colectomies and robotic colectomies, were further divided by their anatomical location into right-sided colectomy and left-sided colectomy. Charts were reviewed and the following patient characteristics were compiled: age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), American Society of Anesthesiology (ASA) score, past medical and surgical history, indication for surgery, and tumor staging (using TNM system, if applicable). Perioperative outcomes included operative time (in minutes), operative blood loss (in milliliters), time to return of bowel function (in days), time to discontinuation of patient controlled analgesia (in days), length of stay (in days), and intraoperative or postoperative complications.
Definitions
Left-sided colectomies were defined as left hemicolectomies, sigmoid colectomies, anterior resections, and low anterior resections. Right-sided colectomies were defined as ileocolic resections, right hemicolectomies, and extended right hemicolectomies. The indication for surgery was grouped into one of four categories: diverticulitis, benign tumor, malignant tumor, and other (Crohn’s disease, diverticulosis, arteriovenous malformation, etc.). Operative blood loss totals were collected from the anesthesia record. Intraoperative complications and postoperative complications were obtained from the official operative dictation report and from the patient chart with a minimum 6-month follow-up period.
Statistical analysis
To establish baseline comparability of the two main treatment groups, chi-square testing was employed for categorical variables (i.e., sex) and analysis of variance (ANOVA) was used for continuous variables (i.e., age, BMI). Results were separated by right and left side and were reported as mean, median, standard deviation, and range. SAS Version 9.2 (Cary, NC: SAS Institute, 2002–2008) was used to analyze the data. A P value < 0.05 was considered statistically significant. The Cox proportional hazards regression method was utilized to determine whether the procedure was associated with time to event (i.e., discharge, recovery of bowel function, and patient-controlled analgesia discontinuance). Subjects who did not reach the event by the close of the study were considered censored. Analysis of Covariance (ANCOVA) was used to examine the effects of surgical procedure on operative time and estimated blood loss. Covariates found to be significantly different in the univariate screening were entered into a multivariate analysis to control for their confounding effects. In addition, subgroup analyses were performed to establish any potential differences based on BMI, ASA score, and indication for procedure.
Operative technique
Robot-assisted sigmoid resection
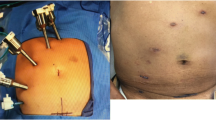
Although there is slight variation based on location of pathology, most left-sided colorectal resections are performed based on eight steps. We will discuss a standardized approach for the robot-assisted sigmoid resection [8]. Under general endotracheal anesthesia in the low lithotomy position, four ports are inserted and pneumoperitoneum is established via the 12-mm umbilical port (Fig. 1). The da Vinci robot is brought in over the patient’s left shoulder and docked to the three upper abdominal ports for mobilization of the splenic flexure. Once the splenic flexure is mobilized, the da Vinci robot is undocked and moved over the patient’s left hip. The patient is then placed in the steep Trendelenburg position, and the robot is redocked to the three lower abdominal ports. The inferior mesenteric artery and vein are dissected using a medial to lateral approach and divided with a 10 mm LigaSure (Covidien, Inc., Dublin, Ireland) or EndoGIA 2.0 vascular stapler via the 12 mm port. The colon is then dissected off the retroperitoneum, and the medial and lateral peritoneal attachments are divided. The mesorectum and rectum are then dissected and divided with an EndoGIA 60 reticulating stapler. The specimen is retrieved through an enlarged umbilical incision, and the anastomosis is created intracorporeally using a circular end-to-end anastomosis (EEA) stapler introduced transrectally. The anastomosis is tested with an air leak test, the ports are removed, and the port sites are closed.
Robot-assisted right hemicolectomy
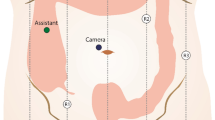
The patient is placed on the operating room table in the supine position, and general endotracheal anesthesia is initiated. Pneumoperitoneum is established via a 12 mm umbilical port and subsequently, an 8 mm suprapubic port, an 8 mm left upper quadrant port, and a 5 mm left lower quadrant port are placed (Fig. 2). The da Vinci robot is brought in over the patient’s right side with the patient in slight Trendelenburg position and right side up and docked to the three upper abdominal ports. The ileocolic artery and vein are dissected free and divided with a 5 mm LigaSure (Covidien, Inc.) or a vascular stapler, and the right colon is mobilized in the medial-to-lateral direction. The mesocolic mesentery is divided to the right of the middle colic vessels, and the right branch is ligated. Before completing the mobilization of the right colon, the gastrocolic and hepatocolic ligaments are freed from their attachments. All pneumoperitoneum is released, and the robot is undocked from its position. The umbilical incision is enlarged, a wound protector is placed, and the entire right colon is exteriorized. A side-to-side functional end-to-end anastomosis is created between the terminal ileum and the proximal transverse colon with a GIA 80 and TA 90 stapler, and the bowel is placed back into the abdominal cavity. The ports are removed and the port sites are subsequently closed.
Results
The demographic data for all 171 patients are compiled in Tables 1 and 2. In the right colectomy group, laparoscopic patients did not differ in age from robotic patients (mean, 70.8 vs. 65.2; P = 0.1516); however, in the left colectomy group there was a statistically significant difference in age (mean, 63.3 vs. 54.6; P = 0.0013), with robotic patients being younger. When looking at BMI and gender, there was no measurable difference between the groups. Based on preoperative ASA score, the laparoscopic right colectomy group had higher ASA class compared with the robotic group (P = 0.0356). Indications for surgery, as one would expect, were more heavily weighted toward benign or malignant tumors for right colectomies and diverticulitis for left colectomies. The specific types of procedures varied but were primarily composed of right hemicolectomies and anterior resections.
The findings from the univariate analysis are compiled in Tables 3 and 4. The results indicate that there is no statistical difference in length of stay, time to bowel function, or time to discontinuation of patient-controlled analgesia between robotic and laparoscopic left and right colectomies. The operating room and procedure time differences between laparoscopic and robotic right colectomies were not statistically significant, whereas there was a measurable difference in the left colectomy group favoring laparoscopy (P < 0.0006). The intraoperative blood loss was significantly lower for right robotic colectomies compared with right-sided laparoscopic procedures (mean, 74.7 vs. 123.9; P < 0.0358). No measurable difference was detected in estimated blood loss for left-sided colectomies. Given the statistically significant discrepancy between the ages of patients undergoing left-sided resections and ASA scores of patients undergoing right-sided procedures, the data were entered into a multivariate analysis. Even after controlling for these variables, the results were no different, showing no statistical difference in length of stay, time to return of bowel function, and time to discontinuation of patient-controlled analgesia.
There were more complications overall (Table 5) in the right colectomy group than in the left colectomy group, with an increased rate of postoperative ileus and bleeding requiring transfusion (10 and 5, respectively) in the laparoscopic cases. The mortality in the study was quite low, with only one death in a patient who underwent a laparoscopic right hemicolectomy. The number of cancer-related resections was disproportionate between the right and left subgroups, and there were differences in the lymph node yields (Table 6). There was a higher yield in robotic right colectomies (21.1 nodes) versus laparoscopic cases (18.7 nodes), but the results were not statistically significant.
A subgroup analysis was performed to test for differences between right and left laparoscopic and robotic colectomies within a given cohort. ASA score was divided into healthy/mild systemic disease (score 1–2) and severe systemic disease (score 3+) to see whether patients with multiple comorbidities did better with a specific surgical modality. BMI was split into underweight, normal, overweight, and obese to evaluate whether the robot had a differential effect on outcome in heavier patients. No significant differences were appreciated in either subgroup analysis. Furthermore, the indications for a procedure were analyzed and subdivided into diverticulitis, tumor, or other. The results did not favor the robotic technology for any specific disease process.
Discussion
Our results indicate that there is no statistical difference in operative time, length of stay, time to return of bowel function, and time to discontinuation of patient-controlled analgesia between robotic and laparoscopic right and left colectomies. There was a statistically significant difference in estimated blood loss in the right-sided colectomy group, with approximately 50 ml less, on average, in robotic cases. Although this may infer the superiority of robotic technology for finer, more precise dissection, such a marginal difference is likely to not be clinically relevant. This small but measurable phenomenon was not seen in the left-sided colectomy group.
The total operating room and procedure time differences between the laparoscopic and robotic colectomies were much smaller than initially anticipated. In the right-sided colectomy group, where undocking the robot is not a requirement, we were able to achieve virtually identical total case- and procedure-specific times between the two groups. However, during left-sided robotic colon resections where robot repositioning was a mandatory step, the surgeon’s ability to reduce operative time is more limited. Our study data, as one would expect, show a significant difference of approximately 30 minutes in favor of the laparoscopic group. Compared with previous studies of similar methodology, our results show an improvement in the right-sided colectomy group of up to an hour and a decrease in time by as much as 40 minutes in the left-sided colectomy cohort (Table 7) [4, 5, 9]. Others have proposed recently performing a completely robotic left-sided colectomy and rectal resection without the need for repositioning the robot, which could potentially decrease operative time even more [10]. As surgeon experience with robotic technology increases, operative times decrease, especially highlighted during right-sided colon resections. Previous studies have looked specifically for the presence of a learning curve, finding it to be approximately 15 to 25 cases [11].
One of the possible limitations of our study was the discrepancy in ASA score in the right-sided colectomy group (P < 0.0356) and age in the left-sided colectomy group (P < 0.0013). This likely represents a surgeon selection bias. At the beginning of the learning curve, younger and healthier patients were more likely to be viewed as candidates for robotic procedures than their older, sicker counterparts. As experience with the robot increased, more difficult cases were being evaluated for the robotic modality. To address this issue, we performed a multivariate analysis controlling for all potential confounders. The results of this calculation also confirmed that there was no statistically significant difference between laparoscopic and robotic colectomy outcomes. We also set out to distinguish the utility of robotic surgery in distinct cohorts of patients, specifically analyzing the outcomes in patients with different BMIs or with a specific indication for surgery (e.g., diverticulitis, cancer). The results of our subgroup analysis did not shed any light on which specific cohort of patients may benefit from this modality. Others have similarly shown the feasibility of robotic surgery in colorectal cancer and diverticular disease but have been unable to establish superiority [10, 12, 13]. Our study does not address the issue of cost, which includes both the initial purchase of the robotic technology as well as ongoing equipment expenditures. At this time, cost considerations certainly weigh in favor of laparoscopy given the current price of robotic technology. However, as the adoption of the robot increases during the next decade, marginal costs will continue to decrease.
Complications were very similar between the laparoscopic and robotic cases, with the exception of postoperative ileus and bleeding, which were higher in the laparoscopic group, although this was not analyzed for statistical significance. These findings are consistent with our belief that robotic surgery allows for more precise, meticulous dissection. Future prospective, randomized trials may help to confirm these findings. The conversion rates to standard laparoscopy were similar in both right and left robotic groups. The two conversions in the right-sided colectomy group were to laparoscopic single-incision right hemicolectomy, both secondary to technical difficulties with the robot. In the left-sided colectomy group, one case was converted to hand assist secondary to difficulty with dissection and the other was converted to open secondary to the extensive nature of the disease process (diverticulitis). Lymph node yields were slightly higher in the right-sided colectomy cohort, although no statistical analysis was performed to assess significance. The findings in the left-sided colectomy group were discordant, with the laparoscopic colectomies producing a considerably higher number of lymph nodes (30 vs. 10). We believe that this discrepancy can be attributed to the way the pathologist analyzed the specimens. The robotic left-sided colectomies were almost entirely performed for diverticular disease and therefore extensive lymph node retrieval was not indicated. Further studies looking specifically at lymph node yields may help to clarify this data.
The usage of the robot in colorectal procedures is still in its infancy and many believe that it has a promising future [14]. Although there are no proven indications at this time, we feel that there may be great intangible benefit. The performance of the robotic colectomy, specifically right hemicolectomy, can help to facilitate competence during the learning curve for other robotic procedures [15, 16]. Furthermore, the robot has been shown to be useful in resident training, easing the transition from open to other forms of minimally invasive surgery [17]. Another area that is largely unexplored is the relationship between a surgeon’s comfort and distractibility and surgical outcomes both currently and over an entire career. It is widely accepted that the ergonomics of laparoscopic surgery are not ideal and can cause various physical ailments [18–20]. We believe that future studies should focus on these measures by surveying minimally invasive surgeons to draw conclusions about the utility of the robot in extending career performance.
There is evidence to support the use of the robot when operating in the pelvis, specifically while performing rectal and low rectal procedures, where dissection and operation is extremely difficult and dangerous [21, 22]. Whereas other studies have validated this conclusion for prostatectomy, they have not confirmed a benefit during proctectomy [23]. In the proximal gastrointestinal tract, certain benefits can be derived when performing a Heller myotomy or gastrectomy, such as lower risk of perforation and shorter recovery time and length of stay [24]. Overall, lack of significant clinical benefits of robotic colorectal surgery over conventional laparoscopic approaches is not surprising. After all, the robotic telemanipulator is simply a tool in the armamentarium of laparoscopic colorectal surgeons that allows for easier performance of the same laparoscopic colon resections commonly performed with conventional instruments.
Conclusions
Our study is one of the largest reviews of robotic colorectal surgery to date. We believe that our results demonstrate the equivalence of robotic surgery to laparoscopic surgery in colorectal procedures. Future research should focus on surgeon-specific variables, such as comfort, ergonomics, distractibility, and ease of use, as other ways to potentially distinguish robotic from laparoscopic colorectal surgery.
References
Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM (2011) A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum 54(2):144–150
Rawlings AL, Woodland JH, Crawford DL (2006) Telerobotic surgery for right and sigmoid colectomies: 30 consecutive cases. Surg Endosc 20(11):1713–1718
Hanly EJ, Talamini MA (2004) Robotic abdominal surgery [review]. Am J Surg 188(4A Suppl):19S–26S
D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D (2004) Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 47(12):2162–2168
Rawlings AL, Woodland JH, Vegunta RK, Crawford DL (2007) Robotic versus laparoscopic colectomy. Surg Endosc 21(10):1701–1708
Delaney CP, Lynch AC, Senagore AJ, Fazio VW (2003) Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46(12):1633–1639
Anvari M, Birch DW, Bamehriz F, Gryfe R, Chapman T (2004) Robotic-assisted laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech 14(6):311–315
DeNoto G, Rubach E, Ravikumar TS (2006) A standardized technique for robotically performed sigmoid colectomy. J Laparoendosc Adv Surg Tech A 16(6):551–556
Spinoglio G, Summa M, Priora F, Quarati R, Testa S (2008) Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum 51(11):1627–1632
Luca F, Cenciarelli S, Valvo M, Pozzi S, Faso FL, Ravizza D, Zampino G, Sonzogni A, Biffi R (2009) Full robotic left colon and rectal cancer resection: technique and early outcome. Ann Surg Oncol 16(5):1274–1278
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860
Ragupathi M, Ramos-Valadez DI, Patel CB, Haas EM (2011) Robotic-assisted laparoscopic surgery for recurrent diverticulitis: experience in consecutive cases and a review of the literature. Surg Endosc 25(1):199–206
D’Annibale A, Pernazza G, Morpurgo E, Monsellato I, Pende V, Lucandri G, Termini B, Orsini C, Sovernigo G (2010) Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol 17(11):2856–2862
Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ (2010) Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis 12(11):1084–1093
deSouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, Abcarian H (2010) Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum 53(12):1611–1617
deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H (2010) Robotic assistance in right hemicolectomy: Is there a role? Dis Colon Rectum 53(7):1000–1006
Huettner F, Dynda D, Ryan M, Doubet J, Crawford DL (2010) Robotic-assisted minimally invasive surgery; a useful tool in resident training–the Peoria experience, 2002–2009. Int J Med Robot 6(4):386–393
Nguyen NT, Ho HS, Smith WD, Philipps C, Lewis C, De Vera RM, Berguer R (2001) An ergonomic evaluation of surgeons’ axial skeletal and upper extremity movements during laparoscopic and open surgery. Am J Surg 182(6):720–724
Stefanidis D, Wang F, Korndorffer JR Jr, Dunne JB, Scott DJ (2010) Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 24(2):377–382
Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D (2010) Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 210(3):306–313
Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H (2010) Robotic colon and rectal surgery: a series of 131 cases. World J Surg 34(8):1954–1958
Ng KH, Lim YK, Ho KS, Ooi BS, Eu KW (2009) Robotic-assisted surgery for low rectal dissection: from better views to better outcome. Singapore Med J 50(8):763–767
Wexner SD, Bergamaschi R, Lacy A, Udo J, Brölmann H, Kennedy RH, John H (2009) The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 23(2):438–443
Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, Plana MN (2010) Efficacy of the da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252(2):254–262
Acknowledgment
The authors thank Myriam Kline, PhD, Department of Biostatistics, Janet Bernstein, Department of General Surgery, and Gerri Ogawa, Medical Records.
Disclosures
Dr. DeNoto is a consultant for Intuitive Surgical, Inc. and Covidien, Inc. and occasionally collects an honorarium for invited lectures. Dr. Deutsch, Dr. Anantha Sathyanarayana, Dr. Gunabushanam, Dr. Mishra, Dr. Rubach, Dr. Zemon, and Dr. Klein have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deutsch, G.B., Sathyanarayana, S.A., Gunabushanam, V. et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc 26, 956–963 (2012). https://doi.org/10.1007/s00464-011-1977-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1977-6