Abstract
Background
The number of patients who have undergone laparoscopic liver surgery has increased in the last 15 years. It is technically challenging surgery, requiring both advanced laparoscopic and liver resection skills. Surgeons often require familiarisation with much of the equipment and techniques used in this type of surgery. No ex vivo model currently exists for laparoscopic liver resection (LLR). The aim of this study was to develop a model for acquiring the technical skills involved in LLR that was also able to assess and measure surgical performance.
Methods
The ProMIS augmented reality surgical simulator was selected because performance data other than time could be obtained, and the simulator was adapted to create the laparoscopic trainer. Twenty candidates with differing laparoscopic surgical experience tested the model. Three groups were identified, novice, intermediate, and expert, according to previous exposure to the laparoscopic tasks. Candidates were required to identify a tumour ultrasonographically, mark and transect ex vivo liver, and perform two laparoscopic stitches with intracorporeal knots. The ProMIS recorded the performance data, including instrument path lengths and time.
Results
Measurements taken from the ProMIS simulator were analysed for statistical differences between the groups. Expert surgeons showed a statistically significant difference in the time taken to identify the liver lesion and transect the organ. The results also demonstrate that the more difficult tasks such as laparoscopic suturing are completed by the expert surgeons with statistically significant shorter times and path lengths compared to the less experienced surgeons.
Conclusion
The adapted ProMIS augmented reality simulator provided junior surgeons with a realistic learning environment in which to familiarise themselves with the equipment and techniques required for LLR. The model also allows assessment of the performance of individuals over time and within a peer group. Construct validity is proven for the suturing component of the model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent years have witnessed the introduction and rapid expansion of laparoscopic surgery. Many operations previously performed as open surgery, including cholecystectomy [1], appendicectomy [2], splenectomy [3], and colectomy [4], have undergone adaptation to allow a minimally invasive approach to be used. The stimulus behind such a change in approach is multifactorial, including the popularity of laparoscopic surgical practice amongst patients. Laparoscopic operations are associated with reduced postoperative pain, shorter hospital stay, and earlier return to work when compared to equivalent operations performed as open surgery [5, 6]. Earlier concerns regarding the safety of laparoscopic surgery when used for the treatment of malignant disease have not been realised [4, 7].
Hepatic surgery is undergoing a similar evolution. Resections of lesions in the anterior liver segments or the left lateral segments can now be performed using a minimally invasive approach. The number of institutions offering such surgery, however, is relatively limited [8]. Laparoscopic liver resection (LLR) requires both advanced laparoscopic skills and liver resection expertise and, thus, has training ramifications. Dissemination of the skills and knowledge that are required for LLR has been relatively slow and generally restricted to consultants and senior trainees [5]. Laparoscopic liver workshops have been set up to train surgeons in the techniques and equipment used in these operations. Such workshops use either human cadavers or live animals as models on which laparoscopic resections can be undertaken. In addition, these workshops are difficult to organise and expensive to run.
The skills required for many minimally invasive operations can now initially be taught using laparoscopic simulators [9, 10]. Considerable effort by industry and the surgical education community has been invested in the design and assessment of these instruments [11]. However, there is no consensus on the timing, type of simulator, and at which stage in a trainee’s career laparoscopic simulation training should be initiated [12]. Nevertheless, laparoscopic simulators are becoming integral parts of the curriculum for surgical trainees around the world [13]. Increasingly, data suggesting a significant improvement in laparoscopic skills following simulation training is emerging [14]. In addition, recent evidence suggests that skills learned on laparoscopic simulators are transferable to the theatre environment [15, 16]. Minimally invasive hepatic surgery also involves the use of many instruments with which junior surgeons in particular may have little or no experience. No virtual reality simulation programme for LLR exists, and to date no other simulator models are available for this type of surgery. The development of a laparoscopic simulator to address the needs of trainee liver surgeons would allow the acquisition of some of the knowledge and skills required to perform LLR in a cost-effective, safe, and reproducible manner.
The aim of this study was to develop a minimally invasive LLR model that would allow trainees to become familiar with some of the equipment and techniques required for laparoscopic liver surgery. The study was also designed to investigate whether the model could identify individuals with differing degrees of laparoscopic expertise from the measurements taken and thus prove its validity.
Methods
Identification of laparoscopic tasks
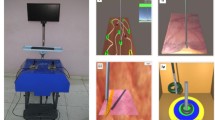
Three distinct tasks with which to assess candidates were identified after considering laparoscopic liver operations. Single tumours in relatively peripheral portions of the liver are the lesions most amenable to laparoscopic resection so this scenario was selected for the model. The first task is identification of the tumour and assessment of the surrounding liver to ensure that it is a solitary lesion. A transection margin is then usually marked with a laparoscopic diathermy hook with a 1-cm margin to ensure complete elimination of the metastasis. In this model lesions are identified with laparoscopic ultrasound equipment, and a resection margin of approximately 1 cm is marked on the liver surface using a marker pen whose ink reservoir is held with a laparoscopic grasper. Once the line of liver transection has been marked, the liver parenchyma is divided using a handheld laparoscopic LigaSureTM device. Division of the liver parenchyma was designated as the second task. The third task was the simulated control of bleeding from the liver. Laparoscopic liver surgery requires the surgeon to be able to control bleeding with laparoscopically inserted stitches. Candidates are required to place stitches at two designated regions on the cut surface of a liver. These stitches are inserted at predetermined regions: an “easy” stitch near the upper surface of the liver, and a “difficult” stitch near the inferior surface of the liver (Fig. 1). The areas to be stitched are 5 mm in diameter, 5 mm from the upper or lower border of the liver surface, and separated on the horizontal plane by 4 cm. A 2/0 prolene stitch cut to a length of 25 cm is used for this task.
Selection of the laparoscopic trainer device
Three laparoscopic simulator models were considered: the simple, low-fidelity box trainer (MISTELS, McGill Inanimate System for Training and Evaluation of Laparoscopic Skills) [9], the high-fidelity virtual reality simulator (LapSim, Surgical Science) [17], and the augmented-reality ProMIS simulator (Haptica) [18]. No virtual reality LLR programme is currently available so this discounted the use of this equipment. The low-fidelity box simulator was similarly discounted because it was not possible to measure objectively candidate performance with factors other than time. The augmented-reality trainer (Fig. 2) uses real objects and instruments with which the candidates perform tasks, but it is also able to track the path of instruments through space using three built-in cameras. Instruments are identified by the ProMIS trainer following the application of black- and yellow-striped tracking stickers placed at a specific distance from the instrument tip. All laparoscopic instruments can have these tracking stickers applied using the measuring tools supplied with the simulator. Using the augmented-reality simulator, a model was realised in which real tissue is used, and calculation of certain metrics (time taken and path length) can be recorded and analysed objectively.
Adaptation of the laparoscopic simulator
Initial experiments using the ProMIS device pointed out problems with the illumination and camera picture. The simulator is lit via two bulbs located at the far end of the machine. As the liver is transected, the organ takes on the shape of an open book. The cut surface of the liver faces away from the bulbs with the transected surfaces in shadow. This, in combination with the dark colour of the liver, means that the cut surface is very poorly illuminated. Not only was this a problem when transecting the liver, but laparoscopic stitching was virtually impossible because the needle could not be seen. Various torches and LED lights were tested in an attempt to increase the illumination of the cut liver surface, but these were either not powerful enough or too bulky to fit inside the simulator. The ProMIS simulator was suitably modified with the addition of a standard 10-mm laparoscope, camera, light source, and monitor. This equipment gave adequate illumination and thus a much improved view of the operative specimen. Using this setup, the ProMIS simulator was able to provide an environment in which the tasks could take place whilst acquiring performance data.
Ex vivo lamb liver was selected for the model. The liver was placed on a mount around which a gauze bandage had been placed. This provided a stable platform for the liver. The liver and mount were placed into a second plastic box to prevent contamination of the simulator. The liver was incised on its inferior surface and a piece of marshmallow confectionary was placed into the liver substance. The confectionary showed up well during ultrasound and also showed obvious deformation by heat if the LigaSureTM transection was not performed at the required distance. Candidates were able to identify the lesion and mark the transection margin on the capsular surface of the liver using the ink reservoir from a marker pen. The transection took place with candidates attempting to adhere to the marked transection margin.
Selection and ranking of candidates
Twenty candidates were selected from a training scheme and consultant surgeon body at a teaching hospital. The participants were allocated into one of three groups: novice, intermediate, and expert. Novices were surgeons who had assisted at or observed laparoscopic procedures but who had little practical laparoscopic exposure. Intermediate candidates were those registrars who had performed laparoscopic procedures such as laparoscopic cholecystectomy, but who rarely performed procedures with intracorporeal knots. Expert status was assigned to surgeons who had extensive laparoscopic experience and who had performed many intracorporeal knots. The same instructions with respect to the task set were given to each candidate as he/she progressed through the model. The laparoscope was held by a third party for each candidate. All candidates were asked about their prior exposure to the equipment used in the study.
Results
The combination of the ProMIS augmented-reality simulator and a standard laparoscopic stack gave candidates a detailed view of the liver and instruments required to perform the tasks. All 20 candidates were able to complete the liver transection (task 2) and laparoscopic suturing (tasks 3 and 4). Unfortunately, seven candidates were unable to complete the task using the laparoscopic ultrasound due to a malfunction of the equipment. The candidates affected by the mechanical breakdown had subsequently moved following their initial assessment and were unable to return to complete the task. The remaining candidates who completed the ultrasound portion of the tasks were able to identify the single lesion in the liver and mark a satisfactory margin for the transection of the liver. For those candidates in the study during the malfunction of the ultrasound, the tumour site was tattooed onto the liver surface. These candidates then marked a 1-cm margin and transected the liver as previously; the other tasks were unaffected.
Interestingly, none of the candidates in the novice group and only four of the seven from the intermediate group had any familiarity with the laparoscopic LigaSureTM device and consequently many found using it particularly difficult. Participants became far more adept with the use of the LigaSureTM instrument as transection across the liver substance was undertaken. All of the candidates were able to completely transect the organ and follow the resection margin marked, and none of the candidates damaged the heat sensitive “tumour.” All candidates completed the stitching tasks.
The results from the tasks varied dramatically and were analysed statistically. The ProMIS simulator recorded both path length (the distance in space that each instrument moved and hence a measure of accuracy and control of the laparoscopic instruments) and the time taken to complete each of the tasks. One-way analyses of variance (ANOVAs) were performed for each of the laparoscopic tasks using GenStat 13th ed. (VSN International Ltd, UK).
There was significant difference between groups in the time taken to identify the “tumour” using laparoscopic ultrasound. Path length in this task was significantly different only between the expert and the intermediate/novice groups (Table 1).
The time taken for transection of the liver was statistically significantly different only between the expert and the novice groups. Path length for this task was significantly shorter for both intermediate and expert groups compared to the novices, though no significant difference was noted between intermediate and expert groups.
There was a statistically significant difference between novice and expert groups for both time and path length for the easy stitch. This was also true between intermediate and expert groups for the time taken. However, there was no significant difference between novice and intermediate groups for either path length or time, and no statistically significant difference in path length between intermediate and expert groups. There was a statistically significant difference for both the time and path length for the difficult stitching task between all groups (Table 2).
Discussion
All the candidates completed the study, although it was interesting that many were unfamiliar with the equipment used in this model. This is remarkable because considerable resources are currently used in the training of junior surgeons. Although this study involved a relatively small number of subjects, it suggests that the use of such ex vivo models may be useful to familiarise surgeons with the ever-increasing equipment and technology that is present in the operating suite. Theatre time is both expensive and a scarce resource. Trainees who are familiar with equipment prior to using it in humans would therefore be advantageous. None of the participants found the projected images difficult to see, suggesting that the modifications to the imaging system of the ProMIS worked satisfactorily. Similarly, the use of a piece of heat-sensitive confectionary was a cheap and effective “tumour.” The tumour could be identified on ultrasound and was not damaged by the transections undertaken. Students tended to be quite conservative with the resection, with many margins being greater than the 1 cm instructed. This is likely to represent some anxiety over the transection margin, with the majority of students being more concerned with complete excision of the lesion.
The transection data demonstrated a statistical difference only for the expert group when compared to novices. This may be explained by the fact that the expert group had the greatest prior exposure to the instrument involved in the task. Associated with this, the absence of a statistically significant difference with respect to time for transection between intermediate and expert groups likely reflects the fact that this was probably the easiest technical exercise in this model. Because the tissue was ex vivo, no bleeding was encountered and participants could progress through the liver substance with little reticence.
The stitching exercise provided some interesting data by which to compare groups. There was no statistically significant difference in time taken for the easy stitch between novice and intermediate groups. Experts, however, completed the task in a significantly shorter time. Interestingly, the path lengths for this task for the expert and intermediate groups were not significantly different. This suggests that the intermediate group acquired the necessary skills to demonstrate economy of movement, but lacked the experience required to reduce the time taken to complete the task. Experts took a significantly shorter time and path length to complete the difficult stitch than did both the novice and intermediate groups. This is also true when comparing intermediate to novice groups. These results are in agreement with those obtained from similar studies in which the more difficult tasks such as suturing demonstrate statistically significant differences when results from individuals with varying abilities were assessed [19, 20]. Such results prove construct validity for the suturing task of the model.
Future improvements to the model centre on the transection and haemorrhage control aspects. It is envisaged that placement of a T-tube connected to a fluid reservoir in the liver at 90° to the transection plane would simulate the transection of a major vein causing “bleeding.” Candidates would have to arrest this “bleeding” with laparoscopic stitches and the metrics of this task could be measured and analysed. The model would be improved because this would introduce an element of stress and also the volume of fluid lost could be measured. This would provide further data with which performances between groups of individuals and single candidates over time could be measured. However, it does add a level of complexity to the setup of the model.
Laparoscopic liver surgery is likely to increase in popularity along with many other minimally invasive operations. Minimally invasive surgery of the liver, however, is technically challenging and requires both advanced laparoscopic skills and expertise in liver resection, and, as with many other minimally invasive procedures, a distinct learning curve is involved [8]. The ProMIS augmented-reality simulator was adapted to give surgeons an adequate view of the organ on which to operate whilst also acquiring performance data. The data acquired can be used to assess candidates within a peer group and the change in performance of an individual over time. The use of this model allows many of the skills required for LLR to be gained in an easily repeatable, cost-effective, and safe manner. This simulator may help surgeons climb the learning curve more quickly.
References
Giger UF, Michel JM, Opitz I, Th Inderbitzin D, Kocher T, Krahenbuhl L, Swiss Association of Laparoscopic and Thoracoscopic Surgery (SALTS) Study Group (2006) Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22, 953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg 203:723–728
Kapischke M, Caliebe A, Tepel J, Schulz T, Hedderich J (2006) Open versus laparoscopic appendicectomy a critical review. Surg Endosc 20:1060–1068
Uranues S, Alimoglu O (2005) Laparoscopic surgery of the spleen. Surg Clin North Am 85:75–90
Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR (2008) Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg 248:728–738
Vigano L, Tayar C, Laurent A, Cherqui D (2009) Laparoscopic liver surgery: a systematic review. J Hepatobiliary Pancreat Surg 16:410–421
Wattiez A, Cohen SB, Selvaggi L (2002) Laparoscopic hysterectomy. Curr Opin Obstet Gynecol 14:417–422
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2084 patients. Ann Surg 250:831–841
Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D (2009) The learning curve in laparoscopic liver resection. Ann Surg 250:772–782
Derossis AM, Fried GM, Abrahamowicz M, Sigman HH, Barkun JS, Meakins JL (1998) Development of a model for training and evaluation of laparoscopic skills. Am J Surg 175:482–487
Fried GM, Feldman LS, Vassiliou MC, Fraser SA, Stanbridge D, Gitulescu G, Andrew CG (2004) Proving the value of simulation in laparoscopic surgery. Ann Surg 240:518-525 (discussion 525–528)
Carter FJ, Schijven MP, Aggarwal R, Grantcharov T, Francis NK, Hanna GB, Jakimowicz JJ (2005) Consensus guidelines for validation of virtual reality surgical simulators. Surg Endosc 19:1523–1532
Sutherland LM, Middleton PF, Anthony A, Hamdorf J, Cregan P, Scott D, Maddern GJ (2006) Surgical simulation: a systematic review. Ann Surg 243:291–300
American College of Surgeons, ACS/APDS Surgical Skills Curriculum for Residents. Available at http://www.facs.org/education/surgicalskills.html (accessed 9 December 2009)
Gurusamy KS, Aggarwal R, Palanivelu L, Davidson BR (2009) Virtual reality training for surgical trainees in laparoscopic surgery. Cochrane Database Syst Rev CD006575
Larsen CR, Soerensen JL, Grantcharov TP, Dalsgaard T, Schouenborg L, Ottosen C, Schroeder TV, Ottesen BS (2009) Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 338:b1802
Sturm LP, Windsor JA, Cosman PH, Cregan P, Hewett PJ, Maddern GJ (2008) A systematic review of skills transfer after surgical simulation training. Ann Surg 248:166–179
Surgical Science, The LapSim System. Available at http://www.surgical-science.com. Accessed 9 December 2009
Haptica, The world-leading ProMIS Surgical Simulator. Available at http://www.haptica.com. Accessed 9 December 2009
Duffy AJ, Hogle NJ, McCarthy H, Lew JI, Egan A, Christos P, Fowler DL (2005) Construct validity for the LAPSIM laparoscopic surgical simulator. Surg Endosc 19:401–405
Van Sickle KR, McClusky DA, Gallagher AG, Smith CD (2007) Construct validation of the ProMIS simulator using a novel laparoscopic suturing task. Surg Endosc 19:1227–1231
Acknowledgments
We thank ConMed for the use of the laparoscopic equipment used in conjunction with the ProMIS simulator. We also acknowledge the help of Nicholas Marlow in setting up the ProMIS simulator as well as John Field for his assistance with the statistical analysis.
Disclosures
A. Strickland, K. Fairhurst, C. Lauder, P. Hewett, and G Maddern have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strickland, A., Fairhurst, K., Lauder, C. et al. Development of an ex vivo simulated training model for laparoscopic liver resection. Surg Endosc 25, 1677–1682 (2011). https://doi.org/10.1007/s00464-010-1440-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1440-0