Abstract
Background
Surgical resection is the gold standard for treatment of early-stage lung tumors. Different minimally invasive approaches are currently under investigation: In addition to conventional video-assisted thoracoscopic surgery (VATS), robotic technology with the da Vinci System has emerged over the past 10 years.
Methods
In this series, 26 patients (12 women and 14 men; median age, 65 years) underwent a robotic lobectomy for early-stage lung tumors (clinical stage IA or IB) or centrally located metastases.
Results
The resected lobes included four left upper lobes, six left lower lobes, eight right upper lobes, and eight right lower lobes. Five intraoperative conversions to open thoracotomy were performed due to one major bleeding, two minor bleedings, one variant course of the pulmonary artery, and one extended resection. The postoperative complications included two prolonged air leaks, one colonic perforation, and one atrial fibrillation. The median hospital stay was 11 days (range, 7–53 days). One 30-day mortality (3.8%) occurred due to respiratory failure. The overall median operative time was 228 min (range, 162–375 min). For the first five patients, the posterior approach was used. Thereafter, the authors switched to an anterior approach, thus enabling an easier hilar dissection. Technical modification within this series also included the introduction of a new vessel sealing device.
Conclusion
Robotic lobectomy was proved to be feasible and safe in our initial series in a learning curve setting. Changes in patient positioning and approach as well as technical modifications resulted in shorter operative times. A longer follow-up period and randomized controlled trials are necessary to evaluate a potential benefit over open and conventional VATS approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lung resection by open thoracotomy with hilar and mediastinal lymph node dissection is considered to be the gold standard of surgery for bronchial cancer [1]. The minimally invasive approach for this condition, reported first in the early 1990s by McKenna [2], gradually gained widespread application as so-called VATS (video-assisted thoracic surgery) lobectomy. Currently, an accepted procedure for patients with early-stage lung cancer, VATS has been shown to reduce postoperative pain and to shorten both chest drain duration and hospital stay [3–6]. Moreover, it may facilitate early onset of adjuvant chemotherapy by quicker rehabilitation after surgery [3].
Despite these advantages, the thoracic surgical community still is rather reluctant to apply the VATS approach for non–small-cell lung cancer. In Europe, the percentage of minimally invasive lobectomies remains below 5% [7]. Obviously, VATS has some limitations discouraging its widespread use in major lung resection: two-dimensional vision (2D), difficult hand–eye coordination, and limited maneuverability, especially in small and poorly accessible areas, thus hindering fine dissection and complex three-dimensional (3D) motion sequences in the chest during procedures dealing with large and vulnerable vessels such as the pulmonary artery [8–11].
Robotic surgical technology was developed to overcome some of these general limitations of minimally invasive surgery. In this report, we describe our experience applying the three-arm da Vinci Surgical Robotic System (Intuitive Surgical, Sunnyvale, CA, USA) to a totally endoscopic minimally invasive lobectomy without rib spreading.
Patients and methods
A retrospective analysis of 26 patients who underwent robot-assisted minimally invasive lobectomy was performed. The indication for the operation was either early-stage lung cancer (clinical stage IA or IB in 24 patients) or centrally located metastases from previous colonic cancer with a need for lobectomy (2 patients). Patients were selected preoperatively based on clinical staging, which was performed using contrast-enhanced computed tomography (CT) scan and positron emission tomography (PET). Preoperatively, histologic confirmation of the tumor was achieved for all patients by CT-guided needle biopsy.
The minimally invasive robotic technique is described in detail. Operation time (without system setup time), intra- and postoperative complications, and hospital length of stay (LOS) were recorded for every patient. Patients were followed at 3 months intervals for the first 2 years, every six months up to 5 years and annually thereafter with radiographic tests at every clinical visit. Suspicion of recurrent disease prompted CT-guided needle biopsy and pathologic examination.
The da Vinci Robotic System
The da Vinci operating robot is a telemanipulation system consisting of a master console, a surgical arm cart, and a conventional monitor cart. The surgical arm cart is the manipulator unit with two or three instrument arms and a central arm to guide a two-channel endoscope that provides 3D vision. The surgeon’s movements of the handles at the master console are transmitted to the tip of the robotic instruments. A table side surgeon is present at any time to change robotic instruments and to assist with conventional minimally invasive instruments if needed.
Operative technique
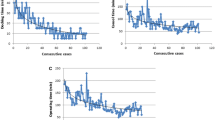
Two different approaches were used in this series (Fig. 1). The first five consecutive operations were performed using a posterior approach. All remaining operations were performed via an anterior approach.
For the posterior approach, patients were placed in a complete lateral decubitus position. Three 2-cm incisions were made, with extension of the inferior incision to 5–7 cm.
For the anterior approach, a partial lateral decubitus position was used. Three incisions were made, one of which was extended. The location of this extended incision depended on tumor location. In the case of upper lobe resection, the lowest incision was extended and vice versa.
The extended incision was used to insert sutures, swabs, suction devices, staplers, and graspers for lung retraction. If needed, an auxiliary incision was made. The camera was placed through the mid trocar. Dissection usually was performed using a Cadiere forceps and a monopolar cautery hook. For blunt dissection, a small round swab was used. Veins, arterial branches, and the bronchus usually were divided using a stapler. For smaller vessels, ligation and stitch ties also were used. Since the introduction of the Hem-o-lok clip (WECK, TFX Medical, High Wycomb, UK) system adapted for robotic use, branches of the pulmonary artery, including larger vessels like the anterior trunk, have been clipped. Occasionally, staplers also have been introduced via additional ports if the angle through one of the three incisions did not allow transection of the vessel or the bronchus.
Using the anterior approach, dissection and division of the hilar structures and division of the fissures for lobectomy were performed as follows:
-
Right upper lobe: upper lobe vein → anterior ascending segmental pulmonary artery (A3/A1) → A2 → upper lobe bronchus → anterior and posterior fissure.
-
Right lower lobe: lower lobe vein → anterior fissure → descending pulmonary artery → A6 → lower lobe bronchus → posterior fissure (can also be performed earlier if necessary).
The posterior approach, used for the first five cases, strictly mimicked the open technique as follows:
-
Dissection and division of the lobular vein → dissection of the fissure anteriorly and posteriorly → preparation and division of the pulmonary arterial branches (upper lobe) or common inferior trunk and A6 (lower lobe) → division of the bronchus.
For left-sided tumors, dissection was performed in the same fashion except for additional dissection of the lingula arteries for left upper lobectomy. No middle lobe lobectomy was performed in this series.
After completion of lobectomy, the robotic arm at the utility incision was removed, and the specimen was taken out using a retrieval bag. Systemic mediastinal lymph node sampling was performed. Staple lines were controlled for air leaks under saline immersion. One or two chest tubes were inserted. Incisions were closed in a standard fashion. All patients were extubated in the operating room.
Statistical analysis
Values are reported as median and range. Students t-test was used to compare the two groups. A p value less than 0.05 was considered statistically significant. Kaplan–Meier analysis was performed to calculate survival. The analysis was performed using SPSS version 11.5 for Windows software (SPSS, Chicago, IL, USA).
Results
A total of 26 robot-assisted minimally invasive lobectomies using the da Vinci Surgical Robotic System have been performed. Patient selection criteria were clinical stage IA or IB tumors or metastases with a need for lobectomy, adequate cardiopulmonary status to tolerate a lobectomy, availability of the da Vinci System and the surgeon performing the procedure, and written informed consent. Clinical staging consisted of CT scans for every patient and PET scans for 20 of 26 patients. Mediastinoscopy was not routinely performed on clinical stage I patients with a PET scan negative for mediastinal lymph nodes.
Median age of the 26 patients (12 women and 14 men) at the time of the operation was 65 years (range, 47–82 years). Right lung lesions predominated (16/26, 61.5%), with an equal distribution of right upper and right lower lobe resections. Fourteen (57.7%) out of 26 tumors were located in the lower lobe. No lobectomy of the middle lobe was performed in this series. The majority of patients had primary lung tumors (non–small-cell lung cancer in 23 patients, low-grade neuroendocrine carcinoma in 1 patient, and lung metastases in 2 patients). Patients’ characteristics are shown in Table 1.
Five procedures (19.2%) were converted to an open thoracotomy. Only one of these (3.8%) was an emergency conversion due to major bleeding from the pulmonary artery with a need for blood transfusion. An arterial tear occurred during extensive manipulation of the vessel because of an adherent lymph node. An emergency thoracotomy was performed, and the bleeding was controlled during conversion by digital compression with a sponge and subsequently managed successfully with a stapling device. All other conversions were due to safety reasons, with two minor bleedings from already dissected lung parenchyma worsening the view, one atypical anatomy of the pulmonary artery, and one case requiring additional parenchymal resection of the adjacent lobe due to the proximity of the tumor. Two of these conversions were in the posterior approach group (40%) and three in the anterior approach group (14.3%).
The median operative time was 228 min (range, 162–375 min). In most cases (21 patients), two chest drains were inserted. Drains were removed if drainage volume was less than 200 ml and no air leak was detectable. Median drainage duration was 7 days (range, 3–15 days), and the median hospital stay was 11 days (range, 7–53 days). Perioperative data are summarized in Table 2.
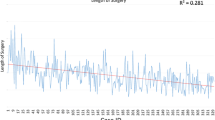
The operative time differed significantly between the posterior and anterior approaches (median, 311 vs 193 min; p = 0.0002; Fig. 2). No difference was found between the two groups in terms of patient characteristics, LOS, or chest drain duration. Concerning pathologic staging, only one upstaging (cT2N0 to pT2N1) was noted in the 24 patients with primary lung tumors.
Complications
No intraoperative or in-hospital mortality occurred. One patient (3.8%) died 1 month after the operation because of respiratory failure under unclear conditions: the patient was discharged after an uneventful postoperative course. Intraoperative bleeding with a need for blood transfusion occurred in our first patient prompting conversion to open thoracotomy. The patient was discharged on postoperative day 13. Two patients (7.6%) had a prolonged air leak for more than 5 days. One patient developed supraventricular tachycardia. Another patient had colonic perforation diagnosed on day 3 after lobectomy and had to undergo an emergency right-sided hemicolectomy. Due to subsequent septic shock syndrome, he was admitted to the intensive care unit (ICU) and had an overall hospital stay of 53 days.
Follow-up
After a median follow-up period of 27 months (range, 1–83 months), 22 patients were alive. Two patients died of cardiorespiratory failure. The one patient experienced a pulmonary embolism 8 months postoperatively, and the other patient experienced respiratory failure on postoperative day 30. Two patients died of tumor recurrence respectively 58 and 18 months after surgery. The one patient had diffuse intrapulmonary and bone metastases, and the other patient had local tumor recurrence 10 months postoperatively. Despite a pathologic stage pT2N1 tumor, the patient refused to undergo adjuvant therapy. Disease-specific survival for all pT1N0 and pT2N0 patients after 12 months was 100% (Fig. 3).
Discussion
Since the introduction of minimally invasive lobectomy in the early 1990s, many different techniques have been proved safe and feasible [5, 6]. Video-assisted lobectomy without rib spreading for minimally invasive lobectomy, currently the standard procedure described in the recent literature, uses one to three port sites with an anterior access incision in the fourth to sixth intercostal space. Absence of rib division or spreading is by definition a requirement for minimally invasive lobectomy [12].
Studies have shown VATS lobectomy to reduce postoperative pain, shorten the LOS, and facilitate early administration of adjuvant chemotherapy [3–6]. However, to date, no large randomized trial demonstrating oncologic equivalency to the standard posterolateral thoracotomy approach in the treatment of non-small-cell lung cancer has been performed. This may be one factor hindering a wider application of minimally invasive lobectomy. Furthermore, any of the different VATS procedures is relatively difficult and requires a certain number of cases to overcome the learning curve [13]. Because of its limited 2D view and restricted maneuverability, technical improvements may facilitate VATS lobectomy.
Robotic technology has been available for more than 10 years. Studies have shown the da Vinci Robotic Surgical System to be effective in performing complex cardiovascular, thoracic, and urologic procedures [14–16]. It provides a stable camera platform with a 3D view for the surgeon, and its Endowrist instruments (Intuitive Surgical, Sunnyvale, CA, USA) allow for seven degrees of freedom with a gain of maneuverability, thus enhancing a surgeon’s ability to identify and dissect anatomic structures [10, 17].
A total of 26 patients underwent robot-assisted lobectomy in our series. The median operative time (228 min) was comparable with that of published series including those of Melfi and Mussi [10] (220 min), Gharagozloo et al. [11] (median, 4 h), and Park et al. [18] (218 min).
The conversion rate was slightly higher than in other published series (Melfi and Mussi [10], 10 of 107 patients; Gharagozloo et al. [11], 0 of 61 patients; Park et al. [18], 4 of 34 patients). The number of conversions can be explained by the number of safety conversions, which in part may have been avoidable: Only one conversion was performed emergently due to massive bleeding of the pulmonary artery. In the literature describing major VATS lobectomy series (100–1,100 patients), the conversion rates for VATS lobectomy range from 0 to 23.5% [19]. As mentioned by McKenna [13], conversion from a minimally invasive approach to a thoracotomy is a step toward patient safety rather than a sign of failure.
The median time for chest drain duration (7 days) was rather long in this series, which can be explained by very defensive drainage management. Drainage duration also had a major impact on LOS (median, 11 days). Other factors influencing LOS included patient preference and reimbursement issues of the Austrian Health System. However, patients were able to leave the hospital without either a drain or a Heimlich valve, and no readmission was required.
Within the first year of the follow-up period, disease-specific survival was 100%, which may support the hypothesis of oncologic equality to the open approach for early-stage lung cancer [20]. However, only long-term results with a longer follow-up period will show the oncologic value of the robotic technique.
After the first three cases, and due to the recognized need for more experience, the lobectomy program was paused for more skills in robotic surgery to be gained through other robot-assisted procedures [15, 21, 22]. Thereafter, we decided to change the positioning of the surgical cart from a posterior to an anterior approach (Fig. 1). In our view, this refinement clearly facilitated dissection of the hilar structures. This hypothesis also was confirmed by shortening of the operation time (posterior vs anterior approach; p = 0.003). The anterior approach allows for improved port placement, thus enabling a larger space for motion and avoiding collision of the robotic arms [18]. In contrast to the posterior approach, the fissures are divided at the end of the operation, diminishing cumbersome parenchymal bleedings.
Other factors influencing the operative time were technical improvements in instrumentation. More recently, instead of ligations or stitch ties, the robot-adapted Hem-o-lok clip was introduced, facilitating transection of vessels. Furthermore, it is important to state that our entire series of robot-assisted lobectomies was performed within a learning curve setting. Thus, in general, the growing experience in robotic surgery also contributed to the improved operative time.
Robotic surgery also has some limitations, the most significant of which is its higher cost. Park et al. [18], identified three major areas of expense: the purchase cost of the da Vinci System, the annual maintenance costs, and the costs of disposable materials. To justify these high expenses, a joint venture between the departments of visceral, thoracic, and transplantation surgery; urology; cardiac surgery; and gynecology and obstetrics was established. By maximizing the use of the robot, the expenses could be shared. As stated by Park et al. [18], the increased use of the da Vinci System over time will continually reduce its cost.
In a survey conducted among members of the European Society of Thoracic Surgeons, Rocco et al. [7] found that more than 58% of the respondents reported performing less than 5% of all their lobectomies by minimally invasive techniques. More than 62% believed that the difficulty of the technique and the steep learning curve hindered a wider application. Robotic thoracic surgery was considered by 59.8% of the respondents to be a part of minimally invasive thoracic surgery and an evolution of video-assisted thoracic surgery. The main reason for its low number of applications remained its high costs.
As long as no randomized controlled trial shows any benefit for robotic surgery, there is no need to purchase the system for minimally invasive thoracic surgery alone. On the other hand, further applications of the robot in minimally invasive thoracic surgery should be evaluated: Whether the robotic system facilitates more complex procedures such as minimally invasive sleeve lobectomies has to be investigated [23].
Based on this series, we can conclude that totally robot-assisted minimally invasive lobectomy is feasible and safe, with a 1-year survival rate of 100% for patients with stage IA or IB lung cancer. Technical improvements such as repositioning of the robotic cart and introduction of new devices will further refine this approach and result in shorter operative times.
Lacking a randomized, controlled design, this series cannot show any advantage of robotic lung lobectomy over conventional posterolateral thoracotomy or VATS lobectomy. Comparisons with recent VATS series also would be critical because robot-assisted lobectomy still is in its infancy. Because VATS lobectomy programs are mostly introduced after fellowships in specialized clinics or proctored training, they may start at a different level of experience and skills than currently reported robotic lobectomy series. Comparisons between robot-assisted and conventional VATS approaches for minimally invasive lobectomies should therefore only be made in institutions with experience in both techniques.
References
Manser R, Wright G, Hart D, Byrnes G, Campbell D (2005) Surgery for early stage non-small-cell lung cancer. Cochrane Database Syst Rev (1):CD004699
McKenna RJ (1994) Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 107:879–881
Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DR, D’Amico TA (2007) Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 83:1245–1249
Grogan EL, Jones DR (2008) VATS lobectomy is better than open thoracotomy: what is the evidence for short-term outcomes? Thorac Surg Clin 18:249–258
Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA (2008) Surgery for early-stage non-small-cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 86:2016–2018
Yan TD, Black D, Bannon PG, McCaughan BC (2009) Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non–small-cell lung cancer. J Clin Oncol 27:2553–2562
Rocco G, Internullo E, Cassivi SD, Van Raemdonck D, Ferguson MK (2008) The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 18:235–247
Dieter RA, Kuzycz GB (1997) Complications and contraindications of thoracoscopy. Int Surg 82:232–239
Bodner J, Wykypiel H, Wetscher G, Schmid T (2004) First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 25:844–851
Melfi FM, Mussi A (2008) Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 18:289–295
Gharagozloo F, Margolis M, Tempesta B (2008) Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 85:1880–1885
Swanson SJ, Herndon JE II, D’Amico TA, Demmy TL, McKenna RJ Jr, Green MR, Sugarbaker DJ (2007) Video-assisted thoracic surgery lobectomy: report of CALGB 39802: a prospective, multi-institution feasibility study. J Clin Oncol 25:4993–4997
McKenna RJ (2008) Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 18:275–280
Wexner SD, Bergamaschi R, Lacy A, Udo J, Brölmann H, Kennedy RH, John H (2009) The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 23:438–443
Augustin F, Schmid T, Bodner J (2006) The robotic approach for mediastinal lesions. Int J Med Robot 2:262–270
Argenziano M, Katz M, Bonatti J, Srivastava S, Murphy D, Poirier R, Loulmet D, Siwek L, Kreaden U, Ligon D, Trial Investigators TECAB (2006) Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 81:1666–1674
Schmid T (2002) Editorial. Eur Surg 34:155–157
Park BJ, Flores RM, Rusch VW (2006) Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 131:54–59
Solaini L, Prusciano F, Bagioni P, di Francesco F, Solaini L, Poddie DB (2008) Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 22:298–310
D’Amico TA (2008) Long-term outcomes of thoracoscopic lobectomy. Thorac Surg Clin 18:259–262
Bodner J, Wykypiel H, Greiner A, Kirchmayr W, Freund MC, Margreiter R, Schmid T (2004) Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 78:259–265
Bodner JC, Zitt M, Ott H, Wetscher GJ, Wykypiel H, Lucciarini P, Schmid T (2005) Robotic-assisted thoracoscopic surgery (RATS) for benign and malignant esophageal tumors. Ann Thorac Surg 80:1202–1206
Ishikawa N, Sun YS, Nifong LW, Chitwood WR, Oda M, Ohta Y, Watanabe G (2006) Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 20:1782–1783
Disclosures
Florian Augustin, Johannes Bodner, Heinz Wykypiel, and Christoph Schwinghammer and have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Augustin, F., Bodner, J., Wykypiel, H. et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 25, 108–113 (2011). https://doi.org/10.1007/s00464-010-1138-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1138-3