Abstract
Introduction/Background
After its initial description in 1990, video-assisted thoracoscopic surgery (VATS) has emerged as the minimally invasive approach for lung resection in early lung cancer.
Methods
A retrospective review of prospectively collected data on patients who underwent robotic pulmonary resection for cancer by a single surgeon, between years 2009 and 2013, was performed. Age, gender, type and duration of surgery, length of stay, estimated blood loss, early and late complications, follow-up time, and local recurrence were reviewed and analyzed descriptively.
Results
Three hundred and thirty-one patients underwent the procedure for pulmonary neoplasm. Two hundred and fifty-nine (79 %) patients underwent anatomic lobectomies, 56 (17 %) patients had wedge resection, while five (1.5 %) patients underwent pneumonectomy. In 11 patients, no pulmonary resection was performed for different reasons. Most common neoplasm was adenocarcinoma (185, 56 %). All procedures involved a systematic mediastinal and hilar lymph node exploration and removal of suspicious nodes. Twenty-six (6.9 %) procedures were converted to open thoracotomy. Mean duration of surgery was 185.63 min. Mean length of hospital stay was 5.52 days. Mean estimated blood loss (EBL) was 47.85 ml. Mean follow-up was 249.41 days (20–1550 days), and five (1.5 %) patients developed local recurrence. Early complications were seen in 29 patients (8.8 %), most commonly cardiac arrhythmias (20, 6 %).
Conclusion
Robotic video-assisted thoracoscopic surgery is feasible in lung lesions, with all the advantages of VATS in terms of decreased length of stay and decreased blood loss with local recurrence rate and complication rate comparable to open procedures. There is a clear need for more studies comparing the apparent advantages of robotic-assisted surgery with increased cost of technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lung cancer is the most common cancer worldwide and leading cause of cancer-related mortality in the USA [1]. Despite multiple treatment modalities from chemotherapy and radiotherapy to surgery, surgical resection remains the only curative treatment for NSCLC [2]. Conventional open thoracotomy with anatomic lobectomy or pneumonectomy has been standard of care for years. The first pulmonary resections by video-assisted thoracoscopic surgery (VATS) were described in 1990s [3]. Since then, a growing body of evidence suggests comparable long-term efficacy and survival with superior postoperative outcome with VATS as compared to conventional thoracotomy [4]. With increased awareness and widespread adoption of CT scans, lung cancer is increasingly being diagnosed at an early stage where minimally invasive surgical resection is appropriate. Despite multiple advantages of minimally invasive approach, there has been a lack of widespread adoption of VATS for pulmonary resection. A review of Society of Thoracic Surgeons General Thoracic Surgery Database showed that VATS approach is used in less than 6–20 % of all lobectomies performed in the USA [5]. The reasons for this limited acceptance of VATS are multifactorial and include restricted vision secondary to 2D nature of conventional laparoscopes, and limited range of motion of instruments due to size and design of these instruments. Further, mediastinal lymph node sampling is also inadequate at times. With technological innovations, robotic video-assisted thoracoscopic surgery (RVATS) has been studied to overcome these limitations [6]. Validation of robotic procedures in many surgical fields has been demonstrated in the literature [7, 8]. Due to the rapid evolution of technology, the paradigm shift toward minimally invasive thoracic surgery has taken place without the support of prospective randomized trials [9]. As pointed out in the literature, such a trial is not feasible at this time. This makes it imperative for the thoracic surgical community to examine all available evidence that can facilitate widespread adoption of minimally invasive pulmonary resection. In this study, we present our experience of robotic VATS performed by a single surgeon in the past 4 years at a community center.
Methods
All patients selected for pulmonary resection underwent an exhaustive preoperative workup including one or more of computed tomography (CT) of chest and abdomen, and/or brain magnetic resonance imaging (MRI) and/or positron emission tomography (PET). Patients with a concern of mediastinal lymph nodes disease in preoperative imaging had preoperative mediastinoscopy and lymph node biopsy. More recently, electromagnetic navigational bronchoscopy with dye tattoo fiducial marking was utilized to overcome tactile limitations for smaller deeper lesions. Pulmonary function tests were routinely utilized to predict the post-op pulmonary function.
Surgical technique
The robotic lung resections were performed initially utilizing the DaVinci Surgical system (Intuitive Surgical, Sunnyvale, CA) with a port-in-port technique. More recently, they are performed utilizing the DaVinci Si system with a 12-mm camera port and two 8-mm metallic ports with upsizing only as needed for stapling device access. A three-arm platform is universally utilized. The robot docks from the patient’s head with anesthesia off to the side. Port configuration routinely is three ports along the 6th or 7th interspace for upper and middle lobe resections and three ports along the 7th or lower interspaces for lower lobe resections. In both cases, a 15-mm access port is placed along the 11th interspace posteriorly for on-field surgical assistance and is enlarged slightly for specimen removal as needed at the completion of the resection. Low-flow carbon dioxide insufflation is universally used. Patient position is critical in the full posterolateral position with maximal flexion to eliminate the patient’s hip from affecting arm or camera mobility. The 30° down camera is routinely used. The 30° up camera is useful for difficult chest wall adhesions. The instruments routinely used include the Cardiere forceps, the curved monopolar shears, the double fenestrated graspers, the DeBakey forceps, and the Maryland bipolar instrument. Once tissue diagnosis is verified, the surgical approach starts with a complete mediastinal lymph node dissection with removal of all enlarged nodes in a counterclockwise nodal station pathway starting at the inferior pulmonary ligament. Once the lymphadenectomy is complete, the venous drainage is routinely divided first. Venous and arterial branches are fully dissected prior to division unless not anatomically feasible or safe. The vascular stapling device of choice is the Endo-GIA curved tip. Parenchymal and bronchial suture lines are most frequently completed using stapling device. Full intercostal nerve blocks are completed with 0.25 % bupivacaine utilizing a 19-gauge needle. The specimen is placed in an endo lap bag for removal via the 11th interspace incision usually by enlarging it one or two centimeters. On-Q pain pumps (I-Flow Corporation, Lake Forest, CA) are routinely used placing the catheters along the neurovascular bundle of the 11th interspace incision. Chest tube drainage is through the camera incision. The patient is repositioned in the supine position, and a chest xray is performed immediately prior to extubation.
Study design
After obtaining approval from Institutional Review Board (IRB) at Mount Sinai Medical Center, we performed a retrospective review of prospectively collected data on patients who underwent robotic pulmonary resection at our medical center by a single surgeon, between years 2009 and 2013. All patients with pre-op staging of “I-II-III A” who underwent robotic pulmonary resection (wedge, lobectomy, or pneumonectomy) were identified. Age, gender, comorbidities, length of surgery, type of surgery, length of stay, estimated blood loss, conversion to open, early (30 days postoperative) and late complications (more than 30 days postoperative), follow-up time, local recurrence, and mortality were reviewed. All the data were analyzed descriptively, using the SPSS software version 22.0.
Results
A total of 331 consecutive patients underwent RVATS with pulmonary resection for malignant pulmonary disease. There was an equal distribution of patients between males and females (male/female = 169: 162). Two hundred and fifty-nine patients (79 %) underwent anatomic lobectomy, 56 patients (17.2 %) underwent wedge resection, while five patients underwent pneumonectomy (1.5 %) (Table 1). All procedures included mediastinal and hilar lymph node exploration, and enlarged and suspicious lymph nodes were resected as described previously. Mean number of lymph node excised was 5.34 (n = 2–22), and mean level of lymph node biopsied was 4.8 (2–11).
The most common neoplasm was adenocarcinoma (185, 56 %), followed by squamous cell carcinoma (44, 13.2 %). Thirty-one patients (9.4 %) had metastatic disease from distant primary sites, and 29 patients had carcinoid tumors (8.8 %). Patients with metastatic tumors were treated with wedge resections, while patients with carcinoid tumors underwent either wedge resection or lobectomy, depending on tumor size and location. All patients with primary lung cancer underwent anatomic lobectomy or pneumonectomy. There were four patients with diagnosis of small cell lung cancer (1.2 %). Two of these patients had indeterminate preoperative and intraoperative pathology, and in other two, the procedures were aborted after intraoperative pathology confirmed the diagnosis (Table 2).
Ten (3 %) patients had only lung biopsy performed secondary to the bulk of the disease or type of intraoperative pathology precluding resection (small cell lung cancer or lymphoma). Twenty-three (6.9 %) procedures were converted to open thoracotomy due to difficult dissection and bleeding. Mean length of ICU stay was 1.62 days, and mean length of hospital stay was 5.53 days (median 4 days). Mean estimated blood loss (EBL) was 47.85 ml (median 50).
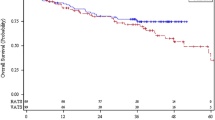
Mean duration of surgery was 185.63 min (median 180.00) (Fig. 1). When we compared the operative time for the first 40 cases with the rest of the patients in the series, there was a clinically significant difference in mean operative time (277.5 min vs. 182.7 min).
Median follow-up was 249.41 days (20–1550 days). Local recurrence of disease was seen in five patients (1.3 %). Four of these patients had undergone wedge resection, while one underwent anatomic lobectomy. The wedge resections were performed for small cell lung cancer, carcinoid, and metastatic melanoma and in one patient with adenocarcinoma who became unstable during the operation, and the procedure was terminated at wedge resection. Early complications were seen in 29 patients (9.3 %), most commonly being supraventricular tachyarrhythmias (20, 6 %). Other complications included chylothorax (2, 0.3 %), pneumonia (3, 0.9 %), bronchopulmonary fistula (1, 0.3 %), and hematoma (1, 0.3 %). Four patients (1.2 %) developed late postoperative complications (more than 30 days post-op), most commonly chronic pain at incision site. Sixty-day mortality rate was 1.2 % (four patients), most commonly secondary to ARDS and sepsis in patients with ASA type 4 (Table 3).
Discussion
Video-assisted thoracoscopic resection was described in 1990s and shown improved results when compared with open thoracotomy with pulmonary resection. It has been shown to be associated with decreased hospital stay, improved postoperative pulmonary function, decreased pain, and lower morbidity [4]. There is a concern regarding the oncologic efficacy of VATS, but a number of studies have shown that local recurrence rates after VATS pulmonary resection are comparable to open thoracotomy and lung resection [4, 10, 11]. In a consensus statement regarding VATS lobectomy by the International Society of Minimally Invasive Cardiothoracic Surgery published in 2007, VATS lobectomy was recommended in clinical stage I and stage II NSCLC patients with no proven difference in 5-year survival compared with open lobectomy [12].
Despite the available literature in support, VATS is not universally applied for surgical management of lung cancer [5]. The reasons are multifactorial and related mostly to ergonomics of the procedure. These include non-optimal visualization, decreased range of motion secondary to bulky instruments, and limited degree of freedom of instruments [13]. Moreover, the extent of mediastinal LN dissection is limited with VATS [9]. RVATS seems a logical extension of VATS in minimally invasive thoracic surgery.
The first case series report on pulmonary resection by RVATS was published in 2002, and a number of subsequent studies have shown encouraging results. Advantages of RVATS include additional four-degree internal yaw, rotation and grip, the elimination of the fulcrum effect, superior 3D vision from binocular camera, reduced human tremor, and improved ergonomic position for the surgeon [14].
In a meta-analysis of retrospective observational studies of RVATS, the perioperative outcome of the procedure was comparable to the results of a systematic view of conventional VATS [4, 12]. A meta-analysis of two retrospective studies with propensity score assessment of perioperative morbidity comparing RVATS with open thoracotomy for early-stage NSCLC, a trend favoring RVATS, was noted [9, 12, 15].
The results of this study compare favorably with the reported results in the literature. In the meta-analysis reported by Cao et al, the perioperative mortality ranged from 0 to 3.8 % which was comparable to our study (1.2 %) [12]. The perioperative morbidity reported thus far ranged from 10 to 39 % while we saw postoperative complications in 9.3 % of our patients [12]. The most common reported complications are tachyarrhythmia (3–19 %), prolonged air leak (4–13 %), pneumonia (1–5 %), and ARDS (1–4 %) [12]. The conversion rate to open thoracotomy was seen in 6.9 % of procedures in our experience, which is similar to historical reports (0–19.2 %) [12].The most common causes of conversion in our experience were adhesions from previous surgery and bleeding. Other reported causes are incomplete fissure, bulky local and mediastinal disease, and non-progression of the case. The average blood loss during the procedure is reported between 30 and 219 ml, while mean blood loss in this study was 47.85 ml. The other advantage reported in the literature is the feasibility of an outstanding N1 and N2 dissections of the mediastinal and hilar lymph node stations using RVATS [9]. The number of lymph node stations dissected ranged from 2 to 11, and average number of lymph node excised was 5.34. That compares favorably with the historical reports (Table 4).
The average operative time in the literature ranges from 132 to 238 min. Mean operative time in our study was 185 min. There is a steep learning curve for RVATS, and operating time has been shown to significantly improve after the initial learning period [14, 18, 19]. Veronesi estimated that the number of operations necessary to attain adequate skill in RVATS to be 20 that was supported by another study [18, 19]. When we evaluated mean operative time in the first 40 procedures with subsequent cases in our study, a statistically significant difference in operative time was noted (277.5 vs. 182.7 min). It has also been felt that early experiences in RVATS were disadvantaged by a lack of standardized surgical technique, limited training opportunities as well as underdevelopment of robotic instrumentation.
Like everything else, RVATS also has its inherent disadvantages. There is an issue of lack of tactile feedback. We have started using electromagnetic navigational bronchoscopy with dye tattoo fiducial marking to overcome tactile limitations for smaller deeper lesions. But the most important and principal issue of RVATS is the associated cost. In one study, Park and Flores reported that RVATS was on average $3981 more expensive than conventional VATS, but $3988 cheaper than open thoracotomy [19]. This is mostly related to the increased cost of the technology and the instruments. Then, there is additional expense of training the nurses and OR staff to assist in RVATS. However, a recent analysis of the voluntary Society of Thoracic Surgery database demonstrated that although the percentage of all lobectomies done by VATS has been increasing, overall only 20 % were performed by VATS during the 3-year study period ending in 2006 [5]. This suggests that the complete adoption rate of VATS lobectomy may in fact be lower in non-academic, community-based settings. If robotic technology can lead to greater adoption of a minimally invasive approach in a safe and appropriate manner, the added cost may be justified by all the attendant benefits over traditional open surgery.
Limitations
This study is limited by the retrospective nature without a matching cohort as control group. But, in today’s day and age, a prospective study with randomization comparing various surgical treatment options for the management of lung cancer is technically not feasible, and inference needs to be drawn from the available literature in form of large case series.
Another limitation of our study is not reviewing costs. The major skepticism about robotic procedure is high overall cost of procedure in comparison with any other types (VATS or open). There is an ongoing debate regarding the cost of the procedure versus the money saved with decrease hospital stay and possibly decreased postoperative complications and blood loss, resulting in less blood transfusion. We feel that if RVATS leads to widespread adoption of minimally invasive surgical treatment for lung cancer, it can offset the cost incurred during the procedure. Cost of procedure and cost-effectiveness was not in scope of this study; yet, such studies in prospective and randomized setting are required to solve this dilemma.
Conclusion
RVATS is feasible in lung tumors, with all the advantages of VATS in terms of decreased length of stay and decreased blood loss with local recurrence rate and complication rate comparable to open procedures. There is a clear need for more studies comparing the apparent advantages of robotic-assisted surgery with increased cost of technology.
References
http://www.cdc.gov/media/releases/2014/p0109-lung-cancer.html. Accessed 20th March 2014
http://www.cancer.gov/cancertopics/pdq/treatment/non-small-celllung/healthprofessional/page4
Walker WS, Carnochan FM, Pugh GC (1993) Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 106:1111–1117
Yan TD, Black D, Bannon PG et al (2009) Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 27:2553–2562
Boffa DJ, Allen MS, Grab JD et al (2008) Data from Society of Thoracic Surgeons General thoracic surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 135:247–254
Ashton RC Jr, Connery CP, Swistel DG et al (2003) Robot assisted lobectomy. J Thorac Cardiovasc Surg 126:292–293
LaPietra A, Grossi EA, Derivaux CC et al (2000) Robotic assisted instruments enhance minimally invasive mitral valve surgery. Ann Thorac Surg 70:835–838
Maeso S, Reza M, Mayol JA et al (2010) Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252:254–262
Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ (2011) Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 142:740–746
Jheon S, Yang HC, Cho S (2012) Video-assisted thoracic surgery for lung cancer. Gen Thorac Cardiovasc Surg. 60(5):255–260
Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW et al (2010) Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 139(2):366–378
Cao C, Manganas C, Ang SC, Yan TD (2012) A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 1(1):16–23
Park BJ (2012) Robotic lobectomy for non-small cell lung cancer (NSCLC): multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 1(1):24–26
Melfi FM, Mussi A (2008) Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 18:289–295
Veronesi G, Galetta D, Maisonneuve P et al (2010) Four arm robotic lobectomy for the treatment of early stage lung cancer. J Thorac Cardiovasc Surg 140:19–25
Radkani P, Joshi D, Barot T, Williams RF (2015) Robotic video-assisted thoracoscopic lung resection for lung tumors: a community tertiary care center experience over four years. Surg Endosc. doi:10.1007/s00464-015-4249-z
Dylewski MR, Ohaeto AC, Pereira JF (2011) Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 23:36–42
Veronesi G, Agoglia BG, Melfi F et al (2011) Experience with Robotic Lobectomy for lung cancer. Innovations 63:355–360
Park BJ, Flores RM (2008) Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 18:297–300
Disclosures
Drs. Pejman Radkani, Tushar Barot, Devendra Joshi, and Roy F. Williams have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radkani, P., Joshi, D., Barot, T. et al. Robotic video-assisted thoracoscopic lung resection for lung tumors: a community tertiary care center experience over four years. Surg Endosc 30, 619–624 (2016). https://doi.org/10.1007/s00464-015-4249-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4249-z