Abstract
Background
Colonic stents are used chiefly for malignant large-bowel obstruction as a palliative measure or bridge to surgery that facilitates one-step resections. Literature on colorectal stenting demonstrates good safety and efficacy; however, a recent trial has raised concerns regarding the safety of a new large-diameter stent, especially in the setting of concurrent chemotherapy. This study evaluated our experience with colorectal stenting using mainly this stent.
Methods
The study was a retrospective chart review with a minimum 6-month telephone follow-up of patients who underwent colorectal stenting for malignant obstruction at Queen’s University between December 2005 and March 2008. The primary outcome was clinical success, defined as full or partial relief of obstructive symptoms or successful bridge to surgery. Clinical failure was defined as persistence or recurrence of obstructive symptoms, death from obstruction, or the need for unplanned surgical intervention.
Results
Thirty patients underwent stenting for malignant obstruction during the study period. The technical success rate was 96.7%. Clinical success was 83% at 30 days and 69% at 6 months. The complication rate was 20%, with four early and two late complications. There were no perforations or stent migrations. Thirty-three percent of patients received chemotherapy with a stent in situ; this was not associated with an increased complication rate. Ninety-one percent of patients and families reported satisfaction with the procedure.
Conclusions
Large-diameter stents appear to be safe for malignant colonic obstruction with and without concurrent chemotherapy and they have similar complication rates as older-generation stents with perhaps lower migration potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enteral self-expanding metal stents (SEMS) present an alternative to surgical management of gastrointestinal obstruction. Since their use was first described in 1991 [1], colonic SEMS have found applications in palliation of malignant strictures, as a bridge to surgery to facilitate one-step resections, and in certain benign obstructions [2]. Although placement of colorectal stents is not without risks, the risks appear to be within the acceptable range. Those most commonly reported include stent obstruction (12%), migration (11%), and the most serious and potentially life threatening—bowel perforation (4%) [3]. Due to their favorable safety and efficacy profile, SEMS have been emerging as the preferred method of treatment for malignant colonic obstruction at experienced centers [4, 5].
However, a recent prospective randomized trial by van Hooft et al. [6] comparing placement of the new large-diameter (25 mm) stent (WallFlex, Boston Scientific, Natick, MA) to surgical resection was prematurely terminated due to an unexpected high rate of major complications in the nonsurgical arm (2 early and 4 late perforations with 3 deaths in 11 patients). These results were surprising because previous reports suggested that the vast majority of perforations occur within 3 days of stent placement and are often related to balloon predilatation of the stricture or caused by the guidewire used to place the stent [7]. A causative role for chemotherapy was proposed because all four late perforations in this trial occurred in patients who received concurrent palliative chemotherapy while having a stent in situ [6]. It was also thought that the design of the stent may have played a role, as the 25-mm WallFlex has a flare with a diameter of 30 mm at its proximal end, which is 8 mm greater than the diameter of the most commonly used old-generation SEMS, the 22-mm WallStent (Boston Scientific).
The aforementioned prospective trial has thus raised concerns regarding the safety of the large-diameter WallFlex, especially in the setting of concurrent chemotherapy [6]. It has led some to question the use of large-diameter stents in any situation [8, 9]. However, a recent study by Repici et al. [10] which evaluated the WallFlex revealed complication rates consistent with those reported for previous generation stents, with only one perforation and no stent-related mortality. Chemotherapy was not offered to patients in this latter study, which may explain the more favorable results. However, since palliative chemotherapy improves survival in advanced colorectal cancer [11], withholding such treatment in patients receiving colorectal SEMS due to fear of late perforation is not an acceptable solution.
Given the limited evidence regarding large-diameter stents and recent safety concerns, we aimed to perform a quality control study of colorectal stenting at our center. Our goal was to evaluate our experience with colorectal stenting using the large-diameter WallFlex, attempt to identify subgroups of patients more prone to complications, and determine the subjective patient experience with stenting.
Methods
This study was a review of patients who underwent colorectal stenting for malignant obstruction at our center between December 2005 and March 2008. Patients were identified using a database of endoscopy suite procedures performed during this period. Every patient undergoing endoscopy at our hospital is entered into this database, which also tracks all interventions (including stenting) that are both attempted and performed. Data on each patient was gathered from procedure records, pathology, radiology, hospital progress and discharge notes, and oncology clinic letters and follow-up reports. In addition, telephone follow-up with patients and patient families was performed to complete data collection and obtain patient and family perspectives regarding the procedure and its outcomes. The study was approved by the Queen’s University Health Sciences and Affiliated Hospitals Research Ethics Board.
All SEMS insertions were performed by one of two experienced endoscopists (LH, 26; DJ, 4). The technique for stent insertion was the same in all cases. Patients with distal obstruction usually underwent no cleansing preparation while those with more proximal obstruction received a high colonic enema. The procedures were done with the patient under conscious sedation with intravenous midazolam and fentanyl given in all but two cases where the patients elected to forego anesthesia. The patients were placed in either the supine or the left lateral decubitus position. All stent insertions were done under fluoroscopic control. A colonoscope was inserted into the rectum and advanced to the point of obstruction, which was usually identified by direct visualization of the tumor. As a rule at our center to reduce risk of stent migration, if the colonoscope could pass through the malignant stricture, stent placement was postponed. A diagnostic ERCP catheter (Balltip Catheter, Boston Scientific) was passed through the lumen of the tumor, usually with the assistance of a guidewire (Hydra Jagwire Guidewire, Boston Scientific) (Fig. 1). Contrast was injected proximal to the tumor to determine the length of the stricture and confirm that the catheter was intraluminal. The SEMS was then deployed across the stricture over the guidewire. Every effort was made to avoid having an end of the stent within a curve in the colon, thus distributing the wall stress along as much the length of colon as possible. This was aided by using of a long stent (12 cm) when possible (Fig. 2). The colonoscope was removed from the patient after injection of contrast confirmed placement and patency of the stent (Fig. 3). Patients were then given a nonstimulant laxative (polyethylene glycol, 125–250 cc/day) for the duration of the stent in place to maintain soft stool and thus avoid impaction at the stent.
Demographic data and various baseline patient characteristics were recorded. Information regarding the intent (palliative vs. bridge to surgery) as well as the result of SEMS placement was collected. It was also noted whether there were any concurrent therapies (chemo- or radiotherapy) after SEMS insertion. Technical data collected included type of preparation (if any), stent model and size, number of stents placed, time to perform the procedure, and amount of sedation. Technical success, defined as SEMS placement bridging the entire stricture with satisfactory endoscopic and fluoroscopic appearance, was determined. Symptom relief at 1 week, 1 month, and 6 months was recorded, as well as time to symptom relief and length of hospital stay. Any complications or stent occlusions were noted along with the subsequent management.
The period of functional patency and need for subsequent interventions were also determined. In the absence of follow-up endoscopy we sought to identify clinical stent migration, which was defined as feeling the stent at the anal orifice, increasing abdominal discomfort requiring further evaluation, or reobstruction with imaging revealing displacement of the stent from its previous position. In cases where death occurred within the study period, the cause of death, presence of stent in situ, and luminal patency at death were noted. Telephone follow-up inquired about patient satisfaction with the procedure, course post-stenting, and present bowel habits or bowel habits prior to death. In order to minimize bias potential, telephone interviews were conducted using a standard questionnaire administered by a nonclinical member of the research team.
The primary outcome of the study was clinical success, defined as full or partial relief of obstructive symptoms, successful bridging to a planned surgical procedure, or successful palliation with SEMS placement and subsequent expiration from a nonobstructive cause. Full relief of symptoms was defined as resolution of all subjective complaints related to the bowel obstruction, as defined by the patient. Partial relief was defined as persistence or occasional recurrence of some subjective symptoms which could be medically managed and did not necessitate further endoscopic or surgical intervention. Clinical failure was defined as no relief of obstructive symptoms, obstructive symptom recurrence, death secondary to bowel obstruction, or requirement for an unplanned surgical intervention.
Results
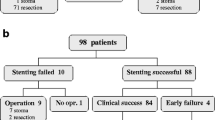
Thirty patients underwent SEMS placement for malignant colorectal obstruction within the study period. Both patients with complete bowel obstruction and those clinically deemed to have impending complete obstruction were considered for SEMS placement. All patients were also assessed by a general surgeon, and due to patient preference and in some cases comorbidities, stenting was chosen as the treatment modality. During this time, colonoscopy was performed in three additional patients with the intention of SEMS placement; however, these patients were deemed not to be candidates for stenting as the colonoscope was able to bypass the strictures in each case and therefore stenting was not attempted. Two of these patients were from other centers and were subsequently lost to follow-up. The third underwent an elective total colectomy 1 month after colonoscopy as he maintained a sufficient luminal diameter to allow for medical management prior to surgery.
Baseline characteristics of the study patients appear in Table 1. Mean patient age was 67 years (range = 39–95 years). Malignancy was advanced in most patients, with 24 (80%) having Stage III or greater disease. The obstruction was in the sigmoid and rectosigmoid areas in 18 (60%) cases. Six cases (20%) involved the right colon. At the time of their presentation, 14 (47%) of the patients were deemed unfit for surgery.
The primary goal of stenting was palliative in 20 patients and bridge to surgery in 10. However, in three cases of bridge to surgery intent, the treatment plan was changed. In two of these cases the patients were found at laparotomy to have an unresectable tumor. In one case no further surgical interventions were performed and the stent became a palliative measure, while in the other case, the surgeon elected to perform a prophylactic ileostomy based on the patient’s favorable life expectancy. A third patient in whom the intent was preparation for primary excision expired before surgery could be performed. Overall, the result of stenting was palliation in 22 patients and a bridge to surgical intervention in 8. Seven of the eight patients in whom the result of stenting was bridge to surgery had elective procedures, while one required emergency surgery due to an ischemic gallbladder perforation that occurred prior to the planned surgery (see Complications). All planned procedures had good bowel preparation and none of the resections were affected by the presence of a stent in situ.
Technical success
The procedural details of SEMS placement appear in Table 2. Thirty-three stents were placed overall, with three cases having long strictures requiring two stents each. Thirty-one of these stents were the 25-mm (large-diameter) WallFlex. The technical success rate was 96.7% (29/30). There was one technical failure where tortuous anatomy was responsible for the inability to pass the wire through the stricture. A second attempt was undertaken the next day in this patient using a pediatric gastroscope to get mid-tumor and pass the wire, after which an adult therapeutic gastroscope was substituted to place two stents across the stricture successfully.
Clinical success
The study outcomes are summarized in Table 3. Clinical success at 7 and 30 days and at 6 months was calculated based on availability of follow-up data. One patient was lost to follow-up and thus excluded from calculations. Clinical success at 7 days was observed in 24 (83%) of the patients available for follow-up. All patients who were a clinical success at 7 days maintained clinical success to 30 days. There were four additional failures after 30 days for a clinical success rate of 69% at 6 months. Two of these failures were due to complications that necessitated surgical intervention, while one patient developed a colo-cutaneous fistula that henceforth acted as a diverting stoma (further discussed below). The last patient had a recurrence of symptoms and died of bowel obstruction after declining further care.
Complications
Complications were divided into early (within 30 days of SEMS placement) and late (after 30 days). There were six complications (4 early, 2 late) for a complication rate of 20%. These were categorized by clinical and pathologic findings as definitely (n = 2), possibly (n = 1), and unlikely (n = 3) stent-related. The complications are summarized in Table 4. One patient underwent SEMS placement as a bridge to surgery. He experienced immediate relief of his obstructive symptoms but unfortunately expired approximately 8 h after endoscopy. Autopsy revealed aspiration pneumonia and a patent, appropriately deployed stent. Therefore, this complication was determined to be a result of obstruction rather than of the stent itself. Another patient suffered an ischemic gallbladder perforation with bile peritonitis 31 days after SEMS placement and was treated with emergency cholecystectomy and right hemicolectomy, afterwards making a full recovery. Pathology failed to reveal colon perforation by the tumor or stent, although the stent was impacted with feces. The pathologist felt that this complication was not stent-related; however, this patient was still classified as a clinical failure. There was one case where a bleeding colo-cutaneous fistula developed through a fungating tumor recurrence at the site of a previous colectomy scar in the anterior abdominal wall. The stent was adjacent to the fistula but shown to be patent and no interventions were performed due to the patient’s extensive intra-abdominal carcinomatosis. The fistula was then treated as a stoma and the stent remained in situ until the patient’s death 1 month later. The contribution of the stent to the development of the fistula is unknown; thus, this complication was classified as possibly stent-related. Finally, there was one case of stenting with palliative intent where surgical excision was undertaken 131 days after SEMS placement due to intractable nausea and vomiting experienced by the patient in the post-stenting course [12].
Ten of the study patients (33%) had concurrent chemotherapy with a stent in situ. One complication (intractable nausea resolving after stent removal, discussed above) was observed in this subgroup. Eight patients had chemotherapy only while two also received radiotherapy to the bowels as part of a high-dose neoadjuvant regimen with 5-FU. The types and mean duration of chemotherapy are summarized in Table 3. Seven of the patients who received concurrent chemotherapy were in the palliative group and three in the bridge-to-surgery group. Mean duration of functional stent patency in patients receiving palliative chemotherapy for whom these data were available was 258 days (n = 6).
There were four stent occlusions (3 definite, 1 possible). Of these, three were in the palliative group and one in the bridge-to-surgery group. Two were due to stool impaction and one was due to tumor growth into the stent after 630 days of functional patency. One occlusion was of an unknown etiology. Mean time to stent occlusion was 242 days.
Long-term follow-up
Telephone follow-up was obtained for 24 (80%) of the cases. Mean time to follow-up was 15.2 months (range = 6–26 months). At follow-up, 21 patients (70%) were deceased, 18 (86%) of whom were stented with palliative intent. Twenty of the nonsurvivors were known to have a stent in situ at death with no follow-up available in the other case. In those who died, mean time to death after stenting was 117 days. Of those patients and families available for follow-up, 91% reported satisfaction with having had the procedure. Many reported an improved sense of well-being and greater enjoyment of daily activities post-stenting. Patients and families also expressed gratitude for the avoidance of surgical interventions including colostomy.
Discussion
The present study suggests an acceptable level of safety and efficacy with large-diameter SEMS placement for malignant colorectal obstruction with and without the use of concurrent chemotherapy. Despite the absence of stringent exclusion criteria, high rates of technical (97%) and clinical success (83%) were seen and are well within the range of those previously reported for colorectal stenting [3, 7]. Palliative SEMS placement eliminated the need for further intervention in the majority of patients, with a colostomy rate of only 14% in this group. In the bridge-to-surgery group, SEMS placement allowed for optimization of functional status prior to surgery as well as time to administer a course of neoadjuvant chemo- and radiotherapy in select patients.
Due to a possible association of late perforation with chemotherapy use, we paid particular attention to this subgroup. Chemotherapy with a large-diameter stent in situ did not appear to be associated with a higher rate of complications. In fact, only one complication was seen in this group, and the duration of functional patency of 258 days was double that for all palliative patients (129 days). Of note, none of our study patients received the VEGF inhibitor bevacizumab (Avastin), which is known to increase risk of gastrointestinal perforation significantly in patients who receive this medication as part of their chemotherapy regimen [13]. It has recently been suggested that SEMS placement may even further increase perforation rates during bevacizumab-based therapy in advanced colorectal cancer, but further studies are required to clarify this [14].
It has been proposed that chemotherapy may increase SEMS migration risk, especially when there is evidence that it has induced tumor shrinkage [7, 10, 15, 16]. Although the sample size in this subgroup was small (n = 10), we did not encounter any clinical stent migrations in this study.
No perforations and no stent-related mortality were observed in our study patients. It has been postulated that the large-diameter WallFlex may carry an increased perforation risk due to pressure necrosis at its proximal end which has a 30-mm-wide flare [9]. To decrease pressure on the bowel wall at any particular point of contact with the stent, we most often elect to use a longer model to distribute wall stress over a greater surface area regardless of stricture length. In addition, every effort is made to avoid placing a stent at a curve or sharp angulation in the colon, which we feel may be a critical technical aspect of stent placement. Of the 31 WallFlex stents placed in the present study, 84% (n = 26) were 12 cm long and the remaining were 9 cm long. In contrast, the van Hooft et al. study [6] used the 12-cm stent only 20% of the time, electing to place the 9-cm stent in 60% of cases and a 6-cm-long model in the remaining 20%. This group did not specify which stent parameters corresponded with the perforations. They did mention that there were no statistically significant associations between stent length and perforation but the absolute numbers were small [6]. However, an association between perforation and stent length or angulation, as opposed to diameter, is speculative at this point and is therefore a factor that warrants further investigation.
We did observe an association between clinical failure and long strictures requiring placement of two stents. Two of three patients who received two stents each were initially clinical failures while the third experienced partial symptom relief but eventually required surgical excision due to persistent nausea and vomiting after stent placement.
The current study does have some limitations. Although retrospective, detailed long-term follow-up allowed us to evaluate the overall clinical role of SEMS placement, with the ability to document both early and late events. Despite the absence of follow-up endoscopy, we were still able to evaluate for all major complications, including clinical perforations, stent-related death, and clinically apparent migration. We were able to evaluate patient and family perception and thus gain a unique insight into how this procedure is received. Although our evaluation of patient and family experience was retrospective and thus vulnerable to bias, it nevertheless suggested a high level of satisfaction with the procedure, which affirms the ultimate clinical role of SEMS placement as minimally invasive and patient-friendly.
In conclusion, this study suggests that large-diameter SEMS with and without concurrent chemotherapy with non-bevacizumab-based regimens are safe for malignant colonic obstruction and have similar complication rates to older-generation stents, including the WallStent, with perhaps a lower migration potential. These results are in contrast with a recent study; this could possibly be explained in part by the length of stents used and stenting technique.
References
Dohmoto M (1991) New method—endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 3:1507–1512
Suzuki N, Saunders BP, Thomas-Gibson S, Akle C, Marshall M, Halligan S (2004) Colorectal stenting for malignant and benign disease: outcomes in colorectal stenting. Dis Colon Rectum 47:1201–1207
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246:24–30
Small AJ, Baron TH (2008) How safe and effective is a nitinol self-expanding metallic stent for palliation of malignant colonic obstruction? Nat Clin Pract Gastroenterol Hepatol 5:356–357
Kozarek R (2008) Making sense out of colonic stents. Gastrointest Endosc 67:85–87
van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA (2008) Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy 40:184–191
Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99:2051–2057
van Hooft JE, Fockens P, Marinelli AW, Bossuyt PM, Bemelman WA (2006) Premature closure of the Dutch stent-in I study. Lancet 368:1573–1574
[No authors listed] (2008) Abstracts from Around the World. Potential hazard of large diameter colonic stent for malignant colonic obstruction. Clin Gastroenterol Hepatol 6:838-839
Repici A, De Caro G, Luigiano C, Fabbri C, Pagano N, Preatoni P, Danese S, Fuccio L, Consolo P, Malesci A, D’Imperio N, Cennamo V (2008) WallFlex colonic stent placement for management of malignant colonic obstruction: a prospective study at two centers. Gastrointest Endosc 67:77–84
Best L, Simmonds P, Baughan C, Buchanan R, Davis C, Fentiman I, George S, Gosney M, Northover J, Williams C (2000) Collaboration Colorectal Meta-analysis. Palliative chemotherapy for advanced or metastatic colorectal cancer. Cochrane Database Syst Rev (2). Art. no. CD001545. doi:10.1002/14651858.CD001545
Mates M, Dudgeon D, Hookey LC, Hurlbut DJ, Belliveau P, Booth CM (2008) Intractable nausea in a patient with metastatic colorectal cancer following insertion of a colonic stent. J Pain Symptom Manage 36:e6–e10
Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10:559–568
Cennamo V, Fuccio L, Mutri V, Minardi ME, Eusebi LH, Ceroni L, Laterza L, Ansaloni L, Pinna AD, Salfi N, Martoni AA, Bazzoli F (2009) Does stent placement for advanced colon cancer increase the risk of perforation during bevacizumab-based therapy? Clin Gastroenterol Hepatol 7:1174–1176
Law WL, Choi HK, Lee YM, Chu KW (2004) Palliation for advanced malignant colorectal obstruction by self-expanding metallic stents: prospective evaluation of outcomes. Dis Colon Rectum 47:39–43
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Disclosures
Ms. Barbara Bielawska, Dr. Lawrence C. Hookey, and Dr. Diederick Jalink have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bielawska, B., Hookey, L.C. & Jalink, D. Large-diameter self-expanding metal stents appear to be safe and effective for malignant colonic obstruction with and without concurrent use of chemotherapy. Surg Endosc 24, 2814–2821 (2010). https://doi.org/10.1007/s00464-010-1055-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1055-5