Abstract
Background
Morbidly obese patients often have impaired respiratory mechanics leading to restrictive and obstructive lung diseases. Weight loss after bariatric surgery has been shown to improve or resolve many obesity-related comorbidities. However, few studies have examined long-term changes in pulmonary mechanics after bariatric surgery. We hypothesize that pulmonary function improves after surgically induced weight loss.
Methods
We examined the pulmonary function of 104 morbidly obese patients who underwent laparoscopic gastric bypass or gastric banding. Pulmonary studies, including forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), and forced expiratory volume at midexpiratory phase (FEV25–75%) were measured preoperatively and at 3-month intervals. All results are expressed as a percentage of the baseline values.
Results
There were 80 females and 24 males with a mean age of 41 years. The mean body mass index was 48 kg/m2. The mean percentage of excess body weight loss at 12 months was 54%. At 12 months postoperatively, restrictive pulmonary mechanics significantly improved as demonstrated by an increase in the FEV1 to 112% of baseline value, increase in the FVC to 109% of baseline value, increase in the PEF to 115% of baseline value, and increase in the FEV25–75% to 130% of baseline value. Additionally, the percentage of patients with obstructive lung pattern (FEV1/FVC ratio less than 0.8) decreased from 9.6% preoperatively to 1.9% postoperatively (p = 0.03).
Conclusions
Weight loss after laparoscopic gastric bypass significantly improves restrictive and obstructive respiratory mechanics. The improvements were observed as early as 3 months postoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bariatric surgery has gained tremendous popularity over the past decade with the introduction of the laparoscopic approach to gastric bypass, development of laparoscopic adjustable gastric banding, and data substantiating the benefits of bariatric surgery and increasing longevity [1–3]. Bariatric surgery has been shown to substantially improve or resolve many obesity-related comorbid conditions, including type II diabetes, hypertension, sleep apnea, and dyslipidemia to name a few. However, few studies have examined the changes in the respiratory mechanics accompanying weight loss from bariatric surgery.
Respiratory function has been shown to decline with increasing body mass index (BMI) and morbidly obese individuals have impaired respiratory mechanics resulting in both restrictive and obstructive lung patterns [4]. It has been postulated that weight loss after bariatric surgery improves pulmonary mechanics as a result of increasing chest wall compliance and reducing airway obstruction [5, 6]. However, few studies have documented this finding with longitudinal follow-up. The aim of this study was to characterize the long-term changes in pulmonary mechanics for morbidly obese patients who underwent laparoscopic gastric bypass or gastric banding.
Materials and methods
All patients being evaluated for surgical treatment of morbid obesity were considered for entry into the study. Patients were considered eligible if they had a BMI between 40 and 60 kg/m2, were less than 60 years of age, and had failed previous nonsurgical attempts at weight loss. Patients who had prior obesity surgery, previous gastric surgery, a large abdominal ventral hernia, or a history of chronic respiratory disease requiring home oxygen were excluded. This study protocol was approved by the Institutional Review Board of the University of California, Irvine Medical Center.
All patients underwent laparoscopic gastric bypass with construction of a 30-mL gastric pouch and a 150-cm Roux limb or laparoscopic adjustable gastric banding. Demographic data, preoperative BMI, and pulmonary history including tobacco use and history of asthma were recorded. Weight loss was measured at 1 month postoperatively and at 3-month intervals. All patients were screened for the diagnosis of sleep apnea using a self-administered questionnaire, the Epworth Sleepiness Scale (ESS). An ESS score of 7 or higher was considered abnormal with a high likelihood of a clinical diagnosis of sleep apnea. Patients with an abnormal score underwent polysomnography to detect for the presence of obstructive sleep apnea and the need for continuous positive airway pressure therapy.
In our patient population, pulmonary function tests are only obtained for patients with a history of smoking, chronic obstructive pulmonary disease, or chronic asthma. However as part of this study, all patients underwent preoperative pulmonary spirometry testing (MicroLab Spirometer, Auburn, ME) performed by a single research assistant. All spirometry measurements were performed with the patient in an upright, sitting position. Each patient was instructed to inhale until their lungs were completely full, seal their lips around the spirometry mouthpiece, exhale as hard and fast as possible until they could not push any more air out, and fully inhale immediately after the expiration maneuver. The best of three spirometric measurements was recorded. Spirometry was repeated in the same fashion on postoperative days 1, 2, and 7, at 1 month, and at 3-month intervals thereafter. Spirometric measurements included forced expiratory volume in 1 sec (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), and forced expiratory volume at midexpiratory phase (FEV25–75%). All spirometric values were reported as a percentage of the baseline values. The FEV1/FVC ratio was also evaluated as an important clinical measurement of airway resistance. A decrease in the FEV1/FVC ratio (less than 0.8) reflects upper airway obstruction due to bronchoconstriction, spasm, airway secretions, or glottic obstruction.
Statistical analysis
All values for continuous variables are expressed as the mean with standard deviation. Repeated measures analysis of variance (ANOVA) were used to analyze pulmonary function studies. After the initial ANOVA, post hoc analysis was performed using paired t-tests to look for significant differences at each time interval compared with baseline values.
Results
Patient demographic and operative data
We examined the pulmonary function of 104 patients who underwent laparoscopic gastric bypass (n = 66) or laparoscopic gastric banding (n = 38). There were 80 females and 24 males. The mean patient age was 41 ± 11 years. The mean preoperative BMI was 48 ± 6 kg/m2. The preoperative spirometric parameters (FEV1, FVC, PEF, and FEV25–75%) are listed in Table 1. There were no conversions to laparotomy. There was no clinical evidence of postoperative pulmonary infection. The percent of excess body weight loss was 54 ± 23% at 12 months.
Pulmonary function
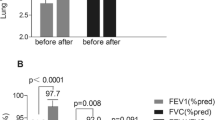
Changes in postoperative respiratory flow parameters are summarized in Figs. 1–4. The FEV1 (Fig. 1) decreased immediately after surgery and returned to within baseline level by postoperative day 7. At 3 months postoperatively, the FEV1 improved above the baseline value. At 12 months postoperatively, the FEV1 measured at 112 ± 16% of baseline value (p < 0.01).
Changes in postoperative respiratory flow of FVC are summarized in Fig. 2. The FVC spirometric parameter decreased immediately after surgery and returned to within baseline level by postoperative day 7. At 3 months postoperatively, the FVC value improved above baseline value. At 12 months postoperatively, the FVC parameter measured at 109 ± 16% of baseline value (p < 0.01).
Changes in postoperative PEF are summarized in Fig. 3. The PEF spirometric parameter decreased immediately after surgery and returned to within baseline level by postoperative day 7. At 1 month postoperatively, the PEF parameter improved above the baseline value. At 12 months postoperatively, the PEF parameter measured 115 ± 25% of baseline value (p < 0.01).
Changes in postoperative FEV25–75% are summarized in Fig. 4. The FEV25–75% spirometric parameter decreased immediately after surgery and returned to within baseline level by postoperative day 7. At 1 month postoperatively, the FEV25–75% parameter improved above the baseline value. At 12 months postoperatively, the FEV25–75% measured 130 ± 27% of baseline value (p < 0.01).
The FEV1/FVC ratio was also evaluated as an important clinical measurement of airway resistance. The percentage of patients with an abnormal FEV1/FVC ratio (less than 0.8) decreased from 9.6% preoperatively to 1.9% postoperatively (p = 0.03).
Discussion
The main finding of this study is that weight loss after laparoscopic gastric bypass or laparoscopic gastric banding for the treatment of morbid obesity resulted in a marked improvement in both the restrictive and obstructive respiratory mechanics. Twelve months after the operation, there was a 12% improvement in the FEV1 parameter, 9% improvement in the FVC parameter, 15% improvement in the PEF parameter, and 30% improvement in the FEV25–75% parameter. Improvement of obstructive disease was documented by reduction in the percentage of patients with abnormal FEV1/FVC ratio (9.6% preoperatively vs. 1.9% postoperatively). These respiratory mechanic improvements occurred as early as 3 months postoperatively. Since morbid obesity impairs respiratory mechanics, improvement of pulmonary function observed after bariatric surgery may be one of the mechanisms for improvement of respiratory conditions associated with morbid obesity.
Lung disorders can be divided into two broad categories of either obstructive or restrictive disorders. With restrictive disorders there is impaired expansion of the thoracic cavity and lungs. With obstructive disorders, there is obstruction to airflow, prolonged expiration, and gas trapping. Morbidly obese individuals tend to have a combination of both obstructive and restrictive disorders. In the morbidly obese, restrictive disease is due to fat accumulation within the thoracic cage and abdominal wall resulting in reduced total respiratory compliance (chest wall and lung parenchyma). Obstructive disease is caused by obstruction of airflow from the adipose tissue deposits within the upper airway and oropharynx or upper glottic obstruction. Clinically, restrictive and obstructive respiratory diseases in the morbidly obese are manifested as dyspnea, daytime somnolence, snoring, fatigue, or the constellation of obstructive sleep apnea and obesity hypoventilation syndrome. Obesity hypoventilation syndrome is diagnosed by an arterial blood gas showing an arterial oxygen tension <55 mmHg and/or a PaCO2 ≥45 mmHg, while obstructive sleep apnea syndrome is diagnosed by polysomnography. Pulmonary function testing is useful for diagnosing lung disease, assessing the severity of the disease, and even measuring responses to therapy. In this study, pulmonary function testing was utilized to measure responses to a surgical therapy (bariatric surgery). The restrictive and obstructive pulmonary impairments in our patient population are not easily detected as the majority of preoperative pulmonary function tests are within normal limits. We observed significant improvement in the pulmonary function with weight loss at long-term follow-up (1 year). The current study is the largest study to show that weight loss associated with bariatric surgery resulted in improvement of both obstructive and restrictive lung impairment. Due to the reduced compliance of the chest wall and lung parenchyma, obese patients may have a restrictive lung impairment that results in a reduction of both the FEV1 and FVC parameters. In this instance, the FEV1/FVC ratio would be normal (greater than 0.8). Unlike restrictive disorder, obstructive airway disorder tends to result in reduction of the FEV1 more than the FVC; hence, the FEV1/FVC ratio is decreased.
Improvement of pulmonary function after bariatric surgery has been observed in several small studies. Santana and colleagues examined changes in pulmonary function test in 39 patients who underwent weight-loss surgery [7]. At a mean 12 months postoperatively, they found a significant increase in both FVC and FEV1 compared with baseline and improvement of pulmonary function was greater in the super-super obese (BMI > 60 kg/m2) compared with morbidly obese patients (BMI < 60 kg/m2). In another small study of 12 patients who underwent laparoscopic adjustable gastric banding, Maniscalco and colleagues reported improvement of the percentage of predicted FEV1 (83% to 87%) and FVC (87% to 95%) at 1 year after the surgery [5]. Marti-Valeri et al. [8] reported 30 patients with improvement of FEV1 (78% to 104%) and FVC (82% to 114%) at 1 year after gastric bypass. Lastly, Davila-Cervantes and colleagues reported that pulmonary function significantly improved in 30 patients who underwent vertical banded gastroplasty [9].
In conclusion, objective findings from this study confirmed that weight loss after bariatric surgery significantly improves both restrictive and obstructive respiratory mechanics associated with obesity. Improvement of postoperative pulmonary function at long-term follow-up is now clearly one of the mechanisms for improvement of clinical conditions such as obstructive sleep apnea and obesity hypoventilation syndrome. Since weight loss in the morbidly obese results in significant improvement in the respiratory mechanics, an important implication from this study is the need for preoperative weight loss in high-risk patients with poor preoperative pulmonary status as this may reduce the patient’s perioperative risk profile.
References
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles A (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737
Sjostrom CD, Lissner L, Wedel H, Sjostrom L (1999) Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS intervention study. Obes Res 7:477–485
Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD (2004) Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 240(3):416–423
Sahbjami H, Gartside PS (1996) Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest 110:1425–1429
Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, Sofia M (2008) Weight loss and asthma control in severely obese asthmatic females. Respir Med 102:102–108
El-Gamal H, Khayat A, Shikora S, Unterborn JN (2005) Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest 128:3870–3874
Santana A, Souza R, Martins AP, Macedo F, Rascovski A, Salge JM (2006) The effect of massive weight loss on pulmonary function of morbid obese patients. Respir Med 100:1100–1104
Marti-Valeri C, Sabate A, Masdevall C, Dalmau A (2007) Improvement of associated respiratory problems in morbidly obese patients after open Roux-en-Y gastric bypass. Obes Surg 17:1102–1110
Davila-Cervantes A, Dominguez-Cherit G, Borunda D, Vargas-Vorackova F, Gonzalez-Barranco J, Herrera MF (2004) Impact of surgically-induced weight loss on respiratory function: a prospective analysis. Obes Surg 14:1389–1392
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, N.T., Hinojosa, M.W., Smith, B.R. et al. Improvement of restrictive and obstructive pulmonary mechanics following laparoscopic bariatric surgery. Surg Endosc 23, 808–812 (2009). https://doi.org/10.1007/s00464-008-0084-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0084-9