Abstract
Background
The effect of extended post-discharge thromboprophylaxis (ETP) on venous thromboembolism (VTE) rates following bariatric surgery (BS) is unknown.
Methods
308 consecutive patients who underwent BS between 2003 and 2007 and who had >1 month of follow-up were included. In-hospital-only VTE prophylaxis (group A), or extended 10-day ETP (group B) was used in 132 and 176 patients, respectively. All patients underwent bilateral lower extremity venous Doppler studies (BLEVDS) prior to discharge. Primary endpoint was the incidence of VTE within 30 days postoperatively. VTE was defined as a clinically evident deep vein thrombosis or pulmonary embolism documented by positive BLEVDS, or computed chest tomography. The primary safety endpoint was bleeding associated with ≥2 g/dL decrease in hemoglobin compared with baseline, transfusion or reoperation.
Results
The incidence of VTE was 1.9% (6/308); 66.6% (4/6) of cases occurred after cessation of thromboprophylaxis. There were no deaths in either group. With the exception of percentage open surgical approach (A: 3% versus B: 0%, p = 0.03), percentage conversions (A: 0 versus B: 3.8%, p = 0.01), and hospital stay (A: 3 versus B: 2.2 days, p < 0.0001), the two groups were comparable in relation to age, sex, body mass index, percentage revision surgery, operative time, and history of VTE. VTE rate was significantly higher in group A (A: 4.5% versus B: 0%, p = 0.006). Although morbidity was higher in group A (A: 12.1% versus B: 1.1%, p < 0.0001), no VTE event occurred in patients who had other complications. The incidence of significant bleeding was lower in group B (A: 5.3% versus B: 0.56%, p = 0.02).
Conclusions
ETP is safe and effective in reducing the incidence of VTE as compared with in-hospital thromboprophylaxis only.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venous thromboembolism (VTE) represents a significant cause of morbidity and mortality among hospitalized patients [1]. Several factors, including age, obesity, abdominal or pelvic surgery, smoking, oral contraceptive use, history of VTE, and venous insufficiency, are associated with increased risk for VTE [2, 3]. Among these risk factors, obesity, defined as a body mass index (BMI) greater than 30 kg/m2, has been a growing problem in the USA, with latest estimates reporting an incidence of 32%, of which 4.8% represent morbid obesity (BMI > 40 kg/m2) [4]. For the morbidly obese individuals, bariatric surgery has been the most durable treatment with an estimated 205,000 procedures performed in the USA in 2007 [5]. VTE is a known complication of bariatric surgery and a significant contributor of the mortality associated with these procedures [6]. As a result, routine perioperative inpatient anticoagulation for thromboprophylaxis after bariatric surgery is currently the standard of care [7]. However, the small number of prospective trials in the bariatric population prevents a definite conclusion about the most effective and safe VTE prophylactic method for obese patients [8]. In addition, although there is evidence to suggest that VTE can occur long after hospital discharge from bariatric procedures [9] and extended post-discharge chemical thromboprophylaxis decreases the risk of VTE after cancer [10] or orthopedic surgery [11], according to a recent position statement from the American Society for Metabolic and Bariatric Surgery there is no consensus with regards to the timing and duration of anticoagulation after bariatric surgery [12]. The principal aim of this study was to assess the impact of the introduction of an extended post-discharge anticoagulation protocol on the VTE rate after bariatric surgery.
Methods
This study includes a single surgeon’s experience of 308 consecutive patients who underwent bariatric surgery between September 2003 and August 2007. Only patients with more than 1 month follow-up were included in the study. No other patient was excluded. Data regarding age, sex, body mass index (BMI), percentage open surgeries, percentage conversions to open surgery, percentage revisions of previous bariatric procedures, percentage of additional procedures performed concomitant with the bariatric surgery, operative time (OT), length of stay (LOS), and 30-day major morbidity were prospectively collected. Additional procedures that were performed concomitant with the bariatric surgery included extensive adhesiolysis for over 30 min, cholecystectomy, oophorectomy, ventral hernia repair with mesh and repair of hiatal, or paraesophageal hernia. Thirty-day major morbidity was defined as the occurrence of death, anastomotic leak, hemorrhage, VTE, small bowel obstruction, cardiac, renal or pulmonary complications within 30 days postoperatively. All patients had at least two pre-existing VTE risk factors: obesity and abdominal surgery. The presence of any additional preoperative risk factors for VTE, such as age greater than 40 years, BMI greater than 60 kg/m2, previous history of VTE, smoking, and documented history of venous insufficiency was also recorded. Oral contraceptives were discontinued for 1 month prior to and after surgery.
VTE surveillance and diagnostic protocol
All patients underwent routinely screening bilateral lower extremity venous Doppler studies (BLEVDS) the day of hospital discharge. VTE was defined as the occurrence of deep vein thrombosis (DVT) of the upper or lower extremities, or pulmonary embolism (PE) or both. VTE diagnosis was made based on strong clinical suspicion combined with positive BLEVDS or helical chest computed tomography.
Thromboprophylaxis protocols and treatment groups
Between September 2003 and December 2005 132/308 (42.9%) patients (group A) received 30 mg enoxaparin sc 1 h prior to surgery followed by enoxaparin 30 mg sc twice a day starting 12 h after surgery for the remaining length of hospital stay. Calf-length intermittent pneumatic compression devices were used routinely in both groups.
Between January 2006 and August 2007 176/308 (57.1%) patients (group B) received enoxaparin 30 mg subcutaneously (sc) twice a day starting 12 h after surgery for the duration of hospital stay followed by a 10-day course of enoxaparin 40 mg sc once a day at home after hospital discharge. Preoperative enoxaparin was not administered. In addition, the gastric remnant staple line was routinely oversewn in this group.
Postoperative prophylactic anticoagulation was discontinued prematurely in patients with signs of significant bleeding. These patients were included in the intention-to-treat VTE analysis.
Primary efficacy and safety endpoints
The primary efficacy endpoint was defined as the incidence of VTE (DVT, PE or both) during the first 30 days after surgery. The safety endpoint was the occurrence of significant postoperative bleeding during the first 30 days after surgery defined as (1) the need for re-exploration, (2) the need for transfusion of blood products, or (3) a hemoglobin (Hb) difference greater than 2 g/dL between the first postoperative Hb and last Hb drawn prior to hospital discharge. Postoperative Hb was routinely obtained in the recovery room, 8–10 h later, and daily thereafter until hospital discharge.
Techniques
Esophagogastroduodenoscopy is performed prior to laparoscopy. Abdominal access is achieved with the Hasson technique, and a 12-mm blunt trocar (Covidien, Norwalk, CT, USA) is placed below the right costal margin. After pneumoperitoneum is established, four 5-mm trocars (Versi-step, Covidien, Norwalk, CT, USA) are placed at the upper abdomen: one below the right costal margin; two at the left upper abdomen in a symmetrical fashion to the right-side ports; and one at the right middle axillary line below the costal margin for the liver retractor (Mediflex Surgical Products, Islandia, NY, USA). Two additional trocars are placed at the periumbilical area; a 12-mm port, 5 cm to the right and a 5-mm port, 3 cm to the left of the umbilicus. A 5-mm 45° camera (Stryker Corp., Kalamazoo, MI, USA) is used.
The pars flaccida is opened with ultrasonically activated coagulating shears (Autosonix, Covidien). A retrogastric window at the lesser sac is created and a 15–30 cc pouch is reconstructed with the 3.5- mm/45-mm linear cutting stapler (EndoGIA II, Covidien). In patients in group B the staple line of the gastric remnant was routinely reinforced with a running “over-and-over” 2.0 Surgidac (Covidien) suture using the Endo-Stitch device (Covidien), paying attention that each suture is placed behind and not through the staple line. Hemostasis at the staple line of the gastric pouch was obtained with clips or interrupted sutures when appropriate. Tissue sealants or staple line reinforcements were not used in any case. The small intestine and its mesentery are divided 50 cm distally from the ligament of Treitz using 3.5-mm and 2.0-mm/45-mm linear cutting staplers, respectively. A retrocolic window is created and the end of the Roux limb is delivered in a retrocolic retrogastric fashion.
The gastrojejunostomy is constructed in a side-to-side fashion with the 3.5-mm/45-mm linear cutting stapler and is measured to be 40 mm in diameter. The gastroenterotomy is closed with a running Connell-type nonabsorbable suture. The gastrojejunostomy is emersed under saline and tested for air leakage using endoscopic insufflation. Using the endoscope as a stent the gastrojejunostomy is circumferentially reinforced with a running Lembert-type nonabsorbable suture.
The jejunojejunostomy is created 150–200 cm distally from the apex of the Roux limb. Two 3.5-mm/60-mm linear cutting staplers are used for the side-to-side anastomosis and the enterotomy closure. A nonabsorbable suture is placed between the apex of the biliopancreatic and Roux limb distally from the anastomosis to prevent kink of the Roux limb. The mesenteric defect is closed with a running nonabsorbable suture. The mesocolic and Petersen’s defects are closed with interrupted U-type nonabsorbable sutures. A closed suction drain is placed behind the gastrojejunostomy and is removed 7 days postoperatively. The fascia of the two 12-mm ports is closed with no. 1 nonabsorbable suture using the Carter–Thomason suture passer. A similar technique was used for the open Roux-en-Y gastric bypass (RYGBP).
Laparoscopic sleeve gastrectomy was performed by resecting the lateral part of the stomach over a 36Fr bougie starting 5 cm proximally from the pylorus along the greater curvature of the stomach. The staple line was reinforced with a continuous suture in a manner similar to laparoscopic RYGBP. Laparoscopic gastroplasty was performed by creation a 15–20 ml gastric pouch and establishment of intestinal continuity with a 25-mm gastrogastrostomy.
We have described previously technical details specific to laparoscopic, or open bariatric revision of vertical banded gastroplasty [13], jejunoileal bypass [14], and Nissen fundoplication [15]. Open or laparoscopic revisions of gastric pouch, jejunostomy, sleeve gastrectomy, or gastric bypass to RYGBP were performed using technical steps similar to those used for the RYGBP. Indications for revision included failure or complications of the original procedure.
Statistical analysis
The paired Student’s t-test or the chi-square tests were used for statistical comparison of continuous or categorical data respectively. p < 0.05 was considered significant. For the relative risk analysis for OT and LOS, respectively, we used a 50% increase of the average OT and an additional day of stay beyond the longest standard LOS (3 days) at any given time during the study. Multivariate regression analysis was not performed because the incidence of VTE was 0 in group B. Instead, post hoc univariate analysis of VTE rates was performed excluding the risk factors with the highest relative risk for VTE and more significant discrepancy between the two groups.
Results
This study includes 255 women and 53 men with an average (range) age and BMI of 43.4 (18–73) years and 46.8 (35–75) kg/m2, respectively. Additional risk factors for VTE aside from morbid obesity and abdominal surgery were present in 83.8% of the patients. One additional risk factor was present in 45.2% of patients, and the frequency of a single patient having two, three, or four risk additional factors was 32%, 6.3%, and 0.3%, respectively. Revision of previous bariatric procedures to RYGBP was performed in 21/308 (6.8%) patients and primary bariatric procedures in the remaining. The mean (range) OT and LOS for the entire patient population were 220.3 (123–505) min and 2.5 (1–14) days, respectively. An open approach was intentionally performed in 4/308 (1.3%) patients. The combined rate of conversion of laparoscopic primary bariatric procedures and laparoscopic revision of bariatric procedures to RYGBP was 2.3% (7/304). Major morbidity rate was 5.8% (18/308). The incidence of VTE in this study was 1.9% (6/308), which was equally divided between DVT and PE. VTE occurred after hospital discharge in 4/6 (66.6%) patients, on postoperative days 12, 20, 26, and 30, respectively. Those four patients had negative BLEVDS prior to hospital discharge. Follow-up BLEVDS at the time of VTE occurrence were positive. Of the two patients who had in-hospital VTE, one had negative BLEVDS and the other, who had symptoms suspicious of VTE, had positive BLEVDS. None of the six patients who developed VTE had suffered from any other major complications. A high relative risk (RR) of VTE was found in association with conversion (RR 20.2) followed by LOS greater than 4 days (RR 8.2), revision surgery (RR 4.8), OT greater than 330 min (RR 3.3), and BMI greater than 60 kg/m2 (RR 3). Two out of the six (33.3%) patients who developed VTE had no additional VTE risk factors other than obesity and abdominal surgery (Table 1).
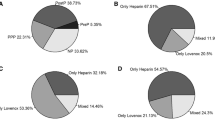
There were no differences between groups A and B in terms of the demographic parameters with the exception of the percentage of open approach and conversions, which were 3% and 3.8%, respectively, in group A and 0 in group B (Table 2). Laparoscopic RYGBP was the predominant bariatric procedure in both groups with comparable distribution (Table 3). In addition, the prevalence of risk factors for VTE (Table 4) and the number of risk factors per patient (Fig. 1) were similar in the two groups except from LOS. The mean LOS was lower in group B (A: 3 versus B: 2.2 days, p < 0.0001). Overall 30-day major morbidity was significantly lower in group B (A: 16/132, 12.1% versus B: 2/176, 1.1%, p < 0.0001), mostly as a result of a decrease in the incidence of significant bleeding and small bowel obstruction. There were no deaths in either group (Table 5).
All patients in group A completed the in-hospital thromboprophylaxis protocol. Three out of 176 (1.7%) patients in group B did not receive chemical thromboprophylaxis. Of these three patients two were Jehovah’s witnesses and one sustained a liver laceration intraoperatively. None of these three patients developed VTE. There was a significant difference in VTE rates between the two groups (A: 6/132, 4.5% versus B: 0, p = 0.006). The significant difference in VTE rates remained in subsequent post hoc analysis where subgroups with higher relative risk for VTE (Table 1) or with significantly different incidence between the two groups (Table 2) were excluded. When the subgroup of patients with conversion (highest relative risk for VTE) was excluded the difference in VTE rates between the two subgroups remained significant (p = 0.03). The incidence of significant bleeding and need for transfusion of blood products were higher in group A (Tables 5 and 6). In contrast, re-exploration rate for bleeding, mean Hb difference, and frequency of Hb difference >2 g/dL were similar in the two groups.
Discussion
In accordance with previous reports [9], the majority of bariatric surgery candidates have at least an additional risk factor for VTE besides obesity and abdominal surgery. Although the presence of additional preoperative risk factors for VTE, such as age greater than 40 years, smoking, and venous insufficiency was frequently observed in this study, only a BMI greater than 60 kg/m2 increased the relative risk of VTE (RR 3). A close relationship between superobesity and PE rates after bariatric surgery has been reported previously as well [16]. Perioperative outcome parameters, however, appear to have a greater effect on VTE rates. According to our findings, conversion to open surgery had the greatest by far impact on VTE occurrence, with a relative risk of 20.2. Conversion rate in this study was 2.3%. Of the seven conversions, three (3/287, 1%) occurred in primary LRYGBP and four (4/21, 19%) in revisions. The relative risk for VTE appears to increase even further when a conversion occurs in a primary laparoscopic RYGBP (RR 35.7) but remains elevated even if the conversion occurs in a laparoscopic revision (RR 3.2). Elevated risk for VTE was also observed with prolonged LOS, revision of previous bariatric surgery, and prolonged OT. In contrast, a history of VTE did not appear to increase the VTE risk.
The VTE rate of 1.9% that we observed in this study falls within the range of 0.8–3.4% previously reported [17, 18]. Similarly, the incidence of PE in this study was 0.95% and in the range of other recently published large series [9, 16]. Although pre- and perioperative risk factors have a significant effect on the incidence of VTE, variation of reported VTE rates between studies may also reflect, to some extent, the aggressiveness of diagnostic surveillance protocol and post-discharge follow-up. All our patients underwent routine screening with BLEVDS prior to discharge and were followed closely thereafter. As a result, two out of six patients with VTE in our study who were diagnosed and treated for VTE in outside institutions were accurately identified and documented.
Our routine surveillance VTE protocol allows us to speculate, with more certainty than previously reported [9, 16, 18], that over half of post-bariatric surgery patients who develop VTE have no clinical symptoms or radiologic evidence of VTE at the time of hospital discharge. The argument that this observation is a result of shorter hospital stay associated with laparoscopic approach is not valid, as post-discharge VTE occurred long after release from hospital in our study. Although venography is the traditional gold standard for diagnosing DVT, BLEVDS are fairly accurate for the diagnosis of asymptomatic DVT in obese patients [18]. Furthermore a large systematic review in asymptomatic postoperative orthopedic patients found that the odds of a positive BLEVDS in the proximal and distal leg veins were 379 and 32 times higher, respectively, among patients with DVT compared with those without [19]. Given the relative accuracy of our diagnostic protocol, the findings in this study suggest that the risk for VTE remains long after hospital discharge and thus post-bariatric surgery patients should be well educated for VTE signs and closely followed the first few weeks postoperatively. Our study also suggests that routine BLEVDS prior to hospital discharge is not indicated outside a research protocol and when suspicious symptoms for VTE are not present.
This study confirms our hypothesis that extended post-discharge chemical thromboprophylaxis (CTP) can decrease significantly the VTE rate after bariatric surgery. In addition, our results indicate that extended CTP may be of more vital importance than the preoperative dose of the chosen anticoagulant. Omission of the preoperative dose of enoxaparin in group B patients did not affect VTE rates, suggesting that it may not have a key role in the prevention of VTE. We believe that our observations are accurate for the following reasons: (1) the patient population in this study represents our entire bariatric experience without the exclusion of any other bariatric cases that we have performed, (2) there were no differences between the two groups in patient selection, operative technique (other than the oversewing of the gastric remnant staple line), or patient management with the exception of the thromboprophylaxis and discharge protocol change as described previously, and (3) the significant benefit of extended thromboprophylaxis on reduction of VTE remained on post hoc analysis where patient subgroups with higher VTE rates or with statistical discrepancy on risk factors between the two groups were excluded.
Nevertheless we acknowledge that the main weakness of our study is that the two treatment groups are not comparable in terms of all VTE risk factors, which despite the results of our post hoc analysis may have contributed to the 4.5% incidence of VTE in group A and the resulting notable difference of VTE rates between the two groups. We would like to address these differences separately. (1) Conversion rate was higher in group A (3.8% versus 0% in group B); it was lower however, or at least comparable to the literature [18, 20], particularly if one takes into consideration that conversions of laparoscopic revision bariatric surgery were included in our study. (2) Group A patients had a longer hospital stay; the difference in mean LOS between the two groups most likely reflects a change in our discharge policy rather than other factors, since the median LOS was 2 days in both groups. (3) Although the percent of patients with revision of previous bariatric surgery was similar in both groups, it is also likely that their inclusion in our study may have contributed to a higher VTE rate, especially if such patients did not receive extended CTP. (4) Complication rate was also significantly higher in group A, but no VTE occurred in such patients. It should again be noted that the morbidity of revision bariatric surgery was included in the 12.1% complication rate observed in group A. The morbidity rate after bariatric surgery in two large studies reporting data from high-volume academic centers [20] and from the state of Pennsylvania discharge database [21] was 16% and 17.4%, respectively. (5) Although the mean operative time was similar in both groups it may be overall longer than reported in other studies [9, 18] and may have contributed to an overall elevated VTE risk. This could be partially attributed to the inclusion of revision bariatric surgery in our study and the high percentage of additional procedures performed in conjunction with the primary bariatric operation. (6) Although there was a difference in the incidence of open approach between the two groups we do not think that it affected our results since none of the four patients who had an intended open approach developed VTE.
Our findings suggest that patients who require conversion of a primary laparoscopic RYGBP should be informed of a substantially higher VTE risk and receive aggressive perioperative thromboprophylaxis. Similar recommendations should probably be extended as well towards patients who undergo revision bariatric surgery, have a BMI greater than 60 kg/m2, or had a prolonged LOS and OT. On the other hand, our observation that 33% of patients with VTE did not have additional risk factors may argue against the practice of limiting the use of additional preventive measures against VTE in “high-risk” patients only, as some patients will still remain at risk and thus suboptimally protected. A larger multicenter study could identify if there are certain subgroups in which extended CTP may not be beneficial.
Our study showed that extended post-discharge CTP is also safe when appropriate surgical techniques are utilized. In contrast to what one would expect, the incidence of significant bleeding was higher in group A than in group B, mostly due to a more frequent need for blood transfusion. This finding is most likely a result of routine oversewing of the gastric staple line and omission of the preoperative dose of anticoagulation in group B patients. We [22], as well as others [23], have shown previously that suture reinforcement of the gastric staple line can significantly reduce bleeding events.
Our results also indicate that duration of treatment may be more important than dosing. Although the choice of prophylactic dose used in our study was arbitrary and not based on patient’s weight or anti-factor-Xa activity it appears to be safe and effective. In contrast, previous studies that used an in-hospital-only VTE prevention protocol based on anti-factor-Xa activity not only still reported a PE rate of 0.4%, but also observed a frequency of perioperative hemorrhagic complications as high as 10.5% [24, 25].
Inferior vena cava (IVC) filter placement is an alternative method of thromboprophylaxis that has been used for high-risk bariatric surgery patients. In our opinion extended CTP alone is a more attractive option for the following reasons. (1) It is a noninvasive method; in contrast, IVC filter placement is invasive with associated morbidity [17] and potentially the additional need for filter retrieval. On some occasions the filter cannot be retrieved, condemning often young patients to live for the rest of their lives with a foreign body and the risk of filter migration [26]. (2) VTE and particularly fatal PE events can still occur, oftentimes in potentially more dangerous sites, such as proximally from the filter [16, 18]; as of today we have not observed any VTE events in patients who received extended, post-discharge thromboprophylaxis. (3) Due to the risk of thrombotic events, it is recommended that patients with an IVC filter should receive anticoagulation as well. In our opinion, that defeats the purpose of the procedure, as extended CTP alone appears to be at least as effective and probably safer. (4) We speculate that extended CTP is probably more cost-effective and thus it can potentially be used in more patients. Randomized trials are required to compare the efficacy of the two modalities for thromboprophylaxis in patients who undergo bariatric surgery.
In conclusion, our findings reiterate previous observations that morbidly obese individuals undergoing bariatric surgery are at substantial risk of developing VTE. In agreement with the existing literature, the majority of bariatric surgery candidates have at least one additional risk factor for VTE besides obesity and abdominal surgery. Furthermore, the routine use of BLEVDS in our study confirms with a higher degree of certainty existing concerns that a significant percentage of VTE associated with bariatric surgery occurs after hospital discharge. As demonstrated herein, the efficacy of extended post-discharge CTP in successfully preventing VTE indicates that duration of thromboprophylaxis may be more important than timing or dosing. To our knowledge, this study represents the first confirmation of the hypothesis, raised by others as well, that extended CTP after bariatric surgery may lead to a reduction of the incidence of VTE.
References
Arnold DM, Kahn SR, Shrier I (2001) Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines. Chest 120:1964–1971
Goldhaber SZ (2004) Pulmonary embolism. Lancet 363:1295–1305
Sapala JA, Wood MH, Schuknecht MP, Sapala MA (2003) Fatal pulmonary embolism after Bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg 13:819–825
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555
Data from the website of the American Society for Metabolic and Bariatric Surgery (ASMBS). Accessed 15 April 2008
Raftopoulos I, Ercole J, Udekwu AO, Luketich JD, Courcoulas AP (2005) Outcome of Roux-en-Y gastric bypass stratified by a body mass index of 70 kg/m2. A comparative analysis of 825 procedures. J Gastrointest Surg 9:44–53
Wu EC, Barba CA (2000) Current practices in the prophylaxis of venous thromboembolism in bariatric surgery. Obes Surg 10(1):7–14
Rocha AT, de Vasconcellos AG, da Luz Neto ER, Araújo DM, Alves ES, Lopes AA (2006) Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg 16(12):1645–1655
Hamad G, Choban PS (2005) Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing Bariatric surgery: findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obes Surg 15:1368–1374
Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nillson PE, Le Moigne-Amrani A, Dietrich-Neto F (2002) Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 346:975–980
Kearon C (2003) Duration of venous thromboembolism prophylaxis after surgery. Chest 124:386S–392S
Position statement (2007) Prophylactic measures to reduce the risk of venous thromboembolism in Bariatric surgery patients. SOARD 3:494–495
Raftopoulos I, Awais O, Courcoulas AP (2007) Laparoscopic revision of open vertical banded gastroplasty to Roux-en-Y gastric bypass. Surg Endosc 21(Suppl 1):S280
Raftopoulos I, Courcoulas A (2008) Revision of jejunoileal bypass to Roux-en-Y gastric bypass. Technical considerations and outcomes from 2 cases. Surg Obes Relat Dis 4(2):198–201
Raftopoulos Y, Awais O, Fernando HC, Courcoulas AP, Luketich JD (2004) Laparoscopic gastric bypass after antireflux operations for the treatment of gastroesophageal reflux disease in morbidly obese patients. Initial experience. Obes Surg 14(10):1373–1380
Carmody BJ, Sugerman HJ, Kellum JM, Jamal MK, Johnson JM, Carbonell AM, Maher JW, Wolfe LG, DeMaria E (2006) Pulmonary embolism complicating Bariatric surgery: detailed analysis of a single institution’s 24-year experience. JACS 203:831–837
Hamad GG, Bergqvist D (2007) Venous thromboembolism in bariatric surgery patients: an update of risk and prevention. SOARD 3(1):97–102
Prystowski JB, Morasch MD, Eskandari MK, Hungness ES, Nagle AP (2005) Prospective analysis of the incidence of deep vein thrombosis in bariatric surgery patients. Surgery 138:759–765
Kassai B, Boissel JP, Cucherat M, Sonie S, Shah NR, Leizorovicz A (2004) A systematic review of the accuracy of ultrasound in the diagnosis of deep vein thrombosis in asymptomatic patients. Thromb Haemost 91:655–666
Nguyen NT, Silver M, Robinson M, Needleman B, Hartley G, Cooney R, Catalano R, Dostal J, Sama D, Blankenship J, Burg K, Stemmer E, Wilson SE (2006) Result of a national audit of bariatric surgery performed at academic centers: a 2004 University HealthSystem Consortium Benchmarking Project. Arch Surg 141(5):445–450
Courcoulas A, Schuchert M, Gatti G, Luketich J (2003) The relationship of surgeon and hospital volume to outcome after gastric bypass surgery in Pennsylvania: a 3-year summary. Surgery 134(4):613–623
Raftopoulos I (2007) Comparison of selective clipping vs. routine oversewing of the gastric remnant staple line on postoperative hemorrhage after laparoscopic Roux-en-Y gastric bypass. A prospective non-randomized comparison trial. Presented at the 24th annual meeting of the American Society for Bariatric Surgery, San Diego, CA
Frezza EE, Wachtel MS (2006) A simple venous thromboembolism prophylaxis protocol for patients undergoing bariatric surgery. Obesity 14(11):1961–1965
Shepherd FM, Rosborough TK, Schwartz ML (2004) Unfractionated heparin infusion for thromboprophylaxis in highest risk gastric bypass surgery. Obes Surg 14(5):601–605
Shepherd MF, Rosborough TK, Schwartz ML (2003) Heparin thromboprophylaxis in gastric bypass surgery. Obes Surg 13(2):249–253
Lorch H, Welger D, Wagner V, Hillner B, Strecker EP, Herrmann H, Voshage G, Zur C, Schwarzbach C, Schröder J, Gullotta U, Pleissner J, Huttner S, Siering U, Märcklin C, Chavan A, Gläser F, Apitzsch DE, Moubayed K, Leonhardi J, Schuchard UM, Weiss HD, Zwaan M (2000) Current practice of temporary vena cava filter insertion: a multicenter registry. J Vasc Interv Radiol 11:83–88
Acknowledgement
The authors would like to thank Bruce Bernstein PhD for his assistance with the statistical analysis in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raftopoulos, I., Martindale, C., Cronin, A. et al. The effect of extended post-discharge chemical thromboprophylaxis on venous thromboembolism rates after bariatric surgery: a prospective comparison trial. Surg Endosc 22, 2384–2391 (2008). https://doi.org/10.1007/s00464-008-0031-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0031-9