Abstract
Background
Studies examining utilization and impact of venous thromboembolism (VTE) chemoprophylaxis for patients undergoing bariatric surgery are limited. Determination of the optimal prophylactic regimen to minimize complications is crucial.
Methods
The Cerner Health Facts database from 2003 to 2013 was queried using ICD-9 codes to identify patients undergoing laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB). VTE chemoprophylaxis regimens were divided into pre-operative alone (PreP), post-operative alone (PostP), both pre-operative and post-operative (PPP), or no prophylaxis (NP). Specific chemoprophylaxis agents were compared. Comparisons in inpatient clinical outcomes were based on univariate analysis and multivariable logistic regression when appropriate.
Results
We identified 11,860 patients who underwent LSG and RYGB. 634 (5.35%) had PreP, 4593 (38.73%) had PostP, 2646 (22.31%) had PPP, and 3987 (33.62%) had NP. The overall rates of transfusion, DVT, and PE were 2.48, 0.27, and 0.18%, respectively. Patients without chemoprophylaxis had higher rate of DVT compared to any chemoprophylaxis (0.58 vs 0.11%, p < 0.0001), without any significant difference in PE rate. Patients with pre-operative chemoprophylaxis were more likely to receive transfusion compared to patients with post-operative prophylaxis alone (OR 1.98, 95% CI 1.28–3), without significant difference in having VTE. When examining heparin versus enoxaparin versus mixed regimen in the PostP group, mixed regimen was associated with increased transfusion requirements (p < 0.001).
Conclusions
Bariatric surgical VTE chemoprophylaxis utilization is inconsistent. In this study, post-operative VTE chemoprophylaxis was associated with decreased VTE events compared to NP, while minimizing bleeding compared to PreP. Mixed therapy using heparin and enoxaparin was associated with more bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venous thromboembolism (VTE) prophylaxis remains a challenging problem in bariatric surgery. This patient population is considered to be at a moderate to high risk of VTE, as patients carry multiple risk factors. Despite attempts to prevent these complications, rates of VTE events following bariatric surgery range between 0.3 and 2.2% [1, 2]. The American College of Chest Physicians (ACCP) recommends either low-molecular-weight heparin, unfractionated heparin, or mechanical prophylaxis for this patient population [3]. However, there is no consensus on standard VTE prophylaxis, timing, dose, or duration. Dosing of anticoagulation can be challenging, as bleeding complications need to be balanced with prevention of deep venous thrombosis or pulmonary embolus.
The objective of this study is to evaluate the optimal VTE prophylaxis in bariatric surgery using a nationwide database. We examined the rates of VTE events and bleeding following surgery. This information is crucial to establish the best practices to minimize complications.
Methods
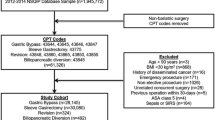
Following Surgery Quality Data Use Group (SQDUG) approval at our institution, the Cerner Health Facts database was searched for all patients undergoing either laparoscopic sleeve gastrectomy (LSG) or laparoscopic Roux-en-Y gastric bypass (RYGB). The Cerner Health Facts® database is a HIPAA-complaint and de-identified database which collects patients’ information from participating facilities. It is a nationwide database of all health facilities which use the Cerner electronic system. It contained data from more than 500 health care facilities in the United States. The database provides comprehensive clinical information about encounter, pharmacy, admission, diagnosis, laboratory orders and results, billing, and procedures for all patient care locations and is time-stamped. The database is longitudinal and hospital patients can be followed post discharge if they return to the same health care system [4]. Patients with surgical ICD-9 procedure codes and CPT codes with associated obesity diagnosis from 2003 to 2013 were included. RYGB and SG patients were identified using ICD-9 diagnosis code for overweight and obesity (ICD-9 codes: 278.00, 278.01, 278.02) and primary procedure codes 44.38, 44.39, 43644, and 43645 for RYGB and 43.89, 43.82, and 43775 for SG. Exclusion criteria included age < 18, duplicate records, history of previous bariatric procedures, and missing surgery date and time.
Prophylaxis was divided into pre-operative (PreP), post-operative (PostP), both pre-operative and post-operative (PPP), and no prophylaxis (NP). Certain variables such as patient demographics and outcomes were examined. Specific prophylaxis agents, enoxaparin and heparin, were compared. ICD-9 diagnosis codes were used to identify transfusion, deep venous thrombosis, and pulmonary embolus were identified during index surgery and within 30 days following the discharge date of index surgery. Confounding medications, such as Warfarin, Fondaparinux, Xarelto, Eliquis, Fragmin, Angiomax, Pradaxa, Acova, Refludan, and Aspirin, were considered.
Chi-square tests with exact p values based on Monte Carlo simulation were utilized to examine the marginal association between categorical variables and clinical outcomes: transfusion, DVT, PE, VTE, 30-day readmission, and 30-day ED visit. Monte Carlo simulation was used because of possible sparse counts in some cells of corresponding contingency tables. Wilcoxon rank-sum tests were used to compare the marginal relationship between any continuous variable and each clinical outcome. Multivariable logistic regression analysis was further built for comparing the risk of having a specific clinical outcome among patients with different DVT prophylaxis. Possible factors that were significantly (p value < 0.1) associated with specific clinical outcome were further considered in the corresponding multivariable logistic regression model. Due to limited number of DVT and PE events, no multivariable logistic regression model was built for three outcomes: DVT, PE, and VTE [5]. We further examined if specific anticoagulation (only heparin, only enoxaparin, and mixed regimens) was associated with several clinical outcomes among the subgroups: 30-day readmission, ED visits, transfusion, DVT, PE, VTE during initial hospital visit, transfusion, DVT, PE, and VTE 30 days following initial hospital visit. Due to the limited sample size, only univariate associations were examined using Chi-square test with exact p value from Monte Carlo simulation. Statistical analysis was performed using SAS 9.4 and the significance level was set at 0.05 (SAS Institute, Inc., Cary, NC, USA).
Results
There were 11,860 records from over 500 hospitals after applying inclusion/exclusion criteria. Majority (88.8%, n = 10,536) of the procedures reported were RYGB, while 11.16% (n = 1,324) were LSG. The rate of 30-day readmission was 4.8%, while the 30-day ED visit was 3.4%. Transfusion was required in 2.48% of patients (n = 294). Eighty-four percent (n = 246) required only one received transfusion. The rate of VTE events was 0.42% (n = 50), with the rate of DVTs being 0.27% (n = 32) and the rate of PE being 0.18% (n = 21).
When examining anticoagulation regimens, 634 patients (5.35%) had PreP, 4593 (38.73%) had PostP, 2646 (22.31%) had PPP, and 3987 (33.62%) had NP (Fig. 1). Among PreP patients, 428 (67.51%) only had Heparin, 130 (20.5%) had only Enoxaparin, and the remaining 11.99% had both. Among PostP patients, 1478 (32.18%) only had Heparin, 2451 (53.36%) had only Enoxaparin, and the remaining 14.46% had both. PPP patients had similar regimens with PreP patients: majority of them (n = 1444, 54.57%) had only Heparin, 559 (21.13%) had only Enoxaparin, and the remaining 24.3% had both.
Certain patient characteristics were significantly different among the different anticoagulation regimen groups, such as age, race, surgery type, facility type, and confounding medication (Table 1). Most academic facilities (43.33%) used only PostP compared to 24.64% of patients from community facilities (p < 0.001). Patients who received confounding medications (n = 1773) were more likely not to receive any anticoagulation (43.6 vs 31.86%, n = 773) or received only PostP (43.32 vs 37.92%, n = 768). No significant differences existed in clinical outcomes (transfusion, DVT, PE, and VTE) with 30 days following discharge based on univariate analysis.
The overall rates of transfusion, DVT, and PE were 2.48, 0.27, and 0.18%, respectively. NP patients had the biggest rates of DVT and VTE (Fig. 2). PPP patients who had 30-day revisits had the biggest rates of transfusion, PE, and VTEs, but this was not significantly different from such rates in other anticoagulation regimen groups (Fig. 3). Patients who had transfusion were more likely to have PreP (10.88 vs 5.2%, p < 0.0001). Patients requiring transfusions were also more likely to have 30-day readmission (10.54 vs 4.68%) and 30-day ED visits (6.12 vs 3.36%, p = 0.0103). However, the rates of DVT, PE, and VTE within 30 days among all revisits were not significantly different between the two groups (transfusion vs no transfusion).
Following multivariable logistic regression, after adjusting for other factors, the risk difference in having transfusion among different regimen groups was significant (p value = 0.02), with patients in the PostP and PPP groups being significantly less likely to have transfusion than patients with only PreP (OR 0.51 with 95% CI 0.33–0.78 and OR 0.64 with 95% CI 0.41–0.99, respectively) (Fig. 4). In addition, patients with age > 45 years, Hispanic patients, and those having DVT or PE were significantly more likely to have transfusion.
Estimated odds ratios and their 95% CI for comparing DVT prophylaxis groups in 30-day readmission, 30-day ED visit, transfusion, DVT, PE, and VTE among different anticoagulation regimen groups. Results were from multivariable logistic regression models for 30-day readmission, 30-day ED visit, and transfusion, and results for DVT, pulmonary embolism, and VTE were based on univariate logistic regression model. Covariates adjusted for 30-day readmission included age, race, region, confounding medication, transfusion, DVT, and pulmonary embolism. Covariates adjusted for 30-day ED visit included age, race, surgery type, region, confounding medication, transfusion, and DVT. Covariates adjusted for transfusion included age, race, surgery type, region, facility type, confounding medication, DVT, and pulmonary embolism
When comparing anticoagulation regimen in terms of the risk of having DVT events, patients with NP were more likely to have DVT than patients with only PostP (OR 4.4, 95% CI 1.8–10.9) or patients with PPP (OR of PPP vs NP = 0.13 with 95% CI 0.03–0.55, Fig. 4). The anticoagulation regimen group was not associated with the risk of having PE (p value = 0.35, Fig. 4).
No significant difference in 30-day readmissions was observed among different VTE prophylaxis groups after adjusting for age, race, region, confounding medication, having transfusion, DVT, or PE (p value = 0.12, Fig. 4). When examining 30-day ED visit, different prophylaxis groups had significantly different risk of 30-day ED visit (p value = 0.0043) after adjusting for age, race, surgery type, region, confounding medication, having DVT or transfusion: patients with NP were less likely to return for ED visits compared to those with only PreP (OR 0.49, 95% CI 0.32–0.75) or those PostP only (OR 0.70, 95% CI 0.54–0.92; Fig. 4).
When further looking at specific pre-operative anticoagulation (only heparin vs only enoxaparin, versus mixed), there was no significant difference among the groups in terms of 30-day readmissions, 30-day ED visits, transfusion, DVT, PE, and VTE in the PreP group. Marginally, medication type was associated with 30-day readmission and transfusion among only the PostP group (p values < 0.0001). Patients having mixed medications were more likely to have transfusion than patients with having only heparin or enoxaparin (4.07 vs 2.50 vs 0.94%, p value < 0.0001). In the PPP group, medication type was associated with 30-day readmissions, VTE, and DVT during follow-up visits (p values = 0.0114, 0.0058, and 0.125, respectively). Mixed medications were significantly associated with a higher incidence of 30-day readmissions than patients having only heparin or enoxaparin (6.53 vs 3.6 vs 5.01%), but with a lower incidence of VTE events during the hospital admission (0.36 vs 3.33 vs 0.83%).
Discussion
Data examining utilization and impact of VTE prophylaxis in bariatric surgery are variable. There is no consensus regarding the type, dose, duration, and timing. Our study further confirms that bariatric surgical VTE prophylaxis is heterogeneous, with patients receiving either of PreP, PostP, PPP, or NP. Further, patients may receive either heparin, enoxaparin, or a mixed regimen (Fig. 1). PostP alone decreases VTE events, while minimizing bleeding without affecting 30-day readmissions. Using mixed medications was associated with more transfusion requirements and 30-day readmissions than with having only heparin or enoxaparin in the PostP group. This can be due to overdosing of medications due to overlapping period of administration. No differences were seen in the PostP group in terms of DVT, PE, or VTE rates among different medication types (either heparin, enoxaparin, or mixed). Medication type also had no effect in the PreP group in terms of VTE events and transfusion, either while hospitalized or in the immediate 30-day period. However, the use of mixed prophylaxis in the PPP group led to an increased number of VTEs while in the hospital and increased 30-day readmissions, whereas using heparin alone caused increased VTE and DVT rates 30 days post-operatively. Enoxaparin use minimized complications, such as DVT and PE in the PPP group, although it was associated with increased need for transfusion, 3.22% for enoxaparin versus 2.49% for heparin and 2.64% for mixed.
Previous studies, examining the rates of VTE in bariatric surgery have reported rates of up to 2.2% despite using chemoprophylaxis [2, 6,7,8]. Our rates of VTE, DVT, and PE within hospital admission post-operatively were 0.42, 0.27, and 0.18, respectively. Rates increased to 0.89, 0.7, and 0.39%, respectively, when assessed up to 30 days post-operatively. Transfusion rates during the hospitalization and 30 days post-operatively were 2.48 and 1.09%, respectively. Winegar showed that a 90-day rate of VTE was 0.42% with majority (73%) of these events occurring after discharge within 30 days. Open procedures were more frequently associated with VTE events (1.54 vs 0.34%) [9]. Finks studied 27,818 patients undergoing primary bariatric surgery between 2006 and 2011 and reported a rate of VTE similar to ours at 0.33% and a PE rate of 0.18%. There was a high mortality associated with these events in their series. Factors such as advanced age, male gender, previous history of VTE, increased operative time, and procedure type were associated with increased risk of VTE events [2]..
Data regarding the timing and type of chemoprophylaxis are variable. While some believe that early ambulation and sequential compression devices may be sufficient [10, 11], others argue the benefits of chemoprophylaxis [12]. Gagner et al. examined data from ten centers in the US and compared patients with sequential compression devices (SCDs) alone versus the addition of chemoprophylaxis between 2005 and 2007, and in 4416 patients. A small portion (9%, n = 396) received SCDs alone. The authors reported an incidence of 30-day VTE of 0.25% in the SCD-alone group compared to 0.47% when anticoagulation was added [10]. The Michigan Bariatric Surgery Collaborative examined data between 2007 and 2012 and reported that VTE rates were lower for low-molecular-weight (LMW) heparin (0.25%, p < 0.001) and unfractionated heparin/LMW (0.29, p < 0.03) compared to the unfractionated heparin group (0.68%) [12]. A meta-analysis of 19 studies showed an incidence of VTE of 0.6% with weight-based chemoprophylaxis, whereas the rate of PE was 0.5 with fixed dose. The use of weight-adjusted heparin dosing was associated with increased bleeding but no decrease in VTE events [13].
Based on our findings of this nationwide multicenter clinical analysis, we recommend using only post-operative prophylaxis to decrease VTE events, while minimizing bleeding with bariatric surgery. Single-agent prophylaxis should be used to decrease bleeding risk. Although not significant, enoxaparin alone had the lowest rate of VTE events up to 30 days post-operatively (0.32 vs 0.84%, when compared to heparin) and may be preferred.
Our study has several limitations. First of all, the Cerner Health Facts database is not designed to collect national representative hospital visit records. However, this database contains data from more than 500 health care facilities across the United States representing millions of patients and its data validity and reliability have been shown [4]. Therefore, findings from this study are highly likely being able to be generalizable and should be viewed as a strong piece of evidence for standardizing DVT prophylaxis for better clinical care. Some of these are associated with using retrospective data obtained from a database, thus having the potential for coding errors. In addition, we cannot distinguish between cause and effect. For example, PreP prophylaxis was associated with increased risk of bleeding. It is possible that this is an effect, as if patients had bleeding, then DVT prophylaxis was not initiated post-operatively. Clinical information, such as the presence of SCDs, body mass index, and information about ambulation, is missing. Although we accounted for confounding medications, we do not have any information regarding when these medications were discontinued pre-operatively, which is a major limitation to this study as this may have had an effect on bleeding. In addition, only symptomatic DVTs were detected, which can lower the rates of DVTs in our analysis.
Conclusion
Bariatric surgical VTE chemoprophylaxis utilization is heterogeneous. Post-operative VTE chemoprophylaxis may decrease VTE events compared to no VTE chemoprophylaxis, while minimizing bleeding compared to pre-operative prophylaxis. The use of mixed prophylaxis (heparin and enoxaparin) post-operatively can be associated with increased bleeding.
References
Stein PD, Matta F (2013) Pulmonary embolism and deep venous thrombosis following bariatric surgery. Obes Surg 23(5):663–668
Finks JF, English WJ, Carlin AM, Krause KR, Share DA, Banerjee M, Birkmeyer JD, Birkmeyer NJ, Michigan Bariatric Surgery Collaborative, Center for Healthcare Outcomes and Policy (2012) Predicting risk for venous thromboembolism with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg 255(6):1100–1104
Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA et al. (2012) Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 Suppl):e227S–e277S
DeShazo JP, Hoffman MA (2015) A comparison of a multistate inpatient EHR database to the HCUP Nationwide Inpatient Sample. BMC Health Serv Res 15:384
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379
Jamal MH, Corcelles R, Shimizu H et al (2015) Thromboembolic events in bariatric surgery: a large multi-institutional referral center experience. Surg Endosc 29(2):376–380
Froehling DA, Daniels PR, Mauck KF et al (2013) Incidence of venous thromboembolism after bariatric surgery: a population-based cohort study. Obes Surg 23(11):1874–1879
MA Bartlett, KF Mauck, PR Daniels (2015) Vasc Health Risk Manag 11:461–477
Winegar DA, Sherif B, Pate V, DeMaria EJ (2011) Venous thromboembolism after bariatric surgery performed by Bariatric Surgery Center of Excellence Participants: analysis of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 7(2):181–188
Gagner M, Selzer F, Belle SH et al (2012) Adding chemoprophylaxis to sequential compression might not reduce risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 8(6):663–670
Frantzides CT, Welle SN, Ruff TM, Frantzides AT (2012) Routine anticoagulation for venous thromboembolism prevention following laparoscopic gastric bypass. JSLS 16(1):33–37
Birkmeyer NJ, Finks JF, Carlin AM, Michigan Bariatric Surgery Collaborative et al (2012) Comparative effectiveness of unfractionated and low-molecular-weight heparin for prevention of venous thromboembolism following bariatric surgery. Arch Surg 147(11):994–998
Becattini C, Agnelli G, Manina G, Noya G, Rondelli F (2012) Venous thromboembolism after laparoscopic bariatric surgery for morbid obesity: clinical burden and prevention. Surg Obes Relat Dis 8(1):108–115
Acknowledgements
We acknowledge the biostatistical consultation and support provided by the Biostatistical Consulting Core at School of Medicine, Stony Brook University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Pryor receives honoraria for speaking for Ethicon, Medtronic, Stryker, and Gore; is a consultant for Medicines Company, Merck, and Intuitive; and received research support from Baronova and Obalon. Drs. Altieri, Yang, Spaniolas, Hajagos, Gasparis, Bates, Docimo, Shroyer, Talamini, and Ms. Park have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Altieri, M.S., Yang, J., Hajagos, J. et al. Evaluation of VTE prophylaxis and the impact of alternate regimens on post-operative bleeding and thrombotic complications following bariatric procedures. Surg Endosc 32, 4805–4812 (2018). https://doi.org/10.1007/s00464-018-6231-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6231-z