Abstract
Background
Barrett’s esophagus–related high-grade dysplasia or mucosal cancer can be treated by endoscopic mucosal resection (EMR), but the adjacent metaplastic epithelium remains at risk for developing further lesions. Our objective was to evaluate the results of the circumferential EMR in removing not only the neoplastic lesion but also the remaining Barrett’s epithelium.
Methods
Forty-one consecutive patients (mean age: 66 years) with Barrett’s esophagus were submitted to 63 EMR sessions in one single-referral endoscopic unit. All patients had high-grade dysplasia, and cancer was detected in 23 of these cases, most of them classified as T1N0 (20 patients) by endosonography. Mucosectomy after saline submucosal injection was performed for the neoplastic lesions and, if necessary, the residual Barrett’s epithelium was removed by the same technique one month later.
Results
A retrospective evaluation showed that, during a mean follow-up of 31.6 months, Barrett’s epithelium was completely replaced by squamous epithelium in 31 (75.6%) cases. There were 10 complications, all of which were managed endoscopically: 8 cases of bleeding and two perforations occurred in 9 (14.3%) patients. One patient developed an esophageal stricture. Barrett’s epithelium recurred in 10 (24.4%) patients and recurrent or metachronous early cancer was detected in 5 (12.2%), all but one of which were treated again by EMR; the fifth patient was referred to surgery. Argon plasma coagulation was used in 6 cases to treat Barrett’s epithelium, and two patients received concomitant chemoradiotherapy as adjuvant therapy.
Conclusions
Circumferential EMR provides an effective endoscopic approach to the management of Barrett’s esophagus-related high-grade dysplasia and mucosal cancer. Additional studies are necessary to evaluate the long-term results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Barrett’s esophagus (BE) can reach an annual incidence of adenocarcinoma up to 70% when high-grade dysplasia is detected in follow-ups of up to 9 years [14, 23] This way, early detection of neoplastic lesions and improvement of endoscopic ablation techniques, especially in those cases not suitable to surgical resection, have become the great priorities in this setting. Endoscopic mucosal resection (EMR) is a good option when the lesion is limited to the mucosa or, as advocated by some authors, to the first third of the submucosa. However, even with a good expectation of cure because of the low probability of locoregional node involvement or distant metastasis in patients with superficial cancers [11], there are still many doubts about the best approach to the treatment of the associated Barrett’s epithelium in order to prevent local recurrence or the development of metachronous lesions The incidence of such lesions in association with Barrett’s esophagus ranges from 14% to 23% [2, 9]. Prevention requires endoscopic surveillance or a combination of endoscopic methods to ablate the remaining Barrett’s epithelium and, in this way, the invisible foci of malignant disease. Nevertheless, pitfalls of the additional methods are the lack of histopathological correlation and the great incidence of strictures, which are noted to occur in up to 30% of patients [1, 15].
We conducted this study to evaluate the efficacy of the complete removal of Barrett’s epithelium by the EMR technique in patients with high-grade dysplasia or mucosal cancer.
Materials and Methods
Between February 1999 and December 2005, 41 consecutive patients with Barrett’s esophagus were referred to EMR of high-grade dysplasia (HGD) or mucosal cancer. The mean length of the Barrett’s epithelium was 4.9 cm (range: 1–15 cm). Endoscopic biopsies had previously detected HGD in all cases, and 23 of these patients presented mucosal carcinoma. Patients with cancer were submitted to endosonographic (EUS) staging with a 5–7.5–10 MHz-radial ultrasonic transducer (EG 36UR or FG36 X; Pentax, Hamburg, Germany) 10–15 days before the first session of EMR. The tumor was classified as usT1 when the third and fifth hyperechoic layers were seen. Overall, based on the pretreatment EUS, the lesions were classified as usT0N0 in one patient and T1N0 in 20 cases. Two additional cases were classified, respectively, as usT2N0 and usT1N1. The option for the endoscopic treatment was based on a marked surgical risk (heart disease, respiratory insufficiency, cirrhosis, or poor general health) or a refusal to surgery.
Circumferential EMR technique
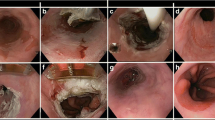
Endoscopic mucosal resection was performed by the same endoscopist (M.G.) according to a procedure described previously [4]. Briefly, the procedure was performed under general anesthesia with propofol (Diprivan® 1%, AstraZeneca Pharmaceuticals, Caponago, Italy), the patient was let in the left side position after an overnight fast. The resection was carried out using either the piecemeal inject-and-cut or the lift-and-cut technique. Most commonly a double-channel endoscope (EG38 UX, Pentax, Hamburg, Germany) was used, but a conventional upper gastrointestinal endoscope (EG40, Pentax, Hamburg, Germany) was also used in some cases. During the first procedure, the index lesion and its contiguous metaplastic tissue were resected. When necessary to avoid the esophageal stricture, the second half of the Barrett’s epithelium was resected one month later. Patients did not receive anything by mouth for 24 h after the procedure and they remained in the hospital for 1–2 days. After the first procedure, every patient received high doses of esomeprazole (80 mg daily) for 30 days, which might have been continued for an additional 4 weeks in case of a second EMR session. After this period, prolonged medical therapy with proton pump inhibitors was recommended. Control endoscopies with Lugol staining and biopsies from the entire length of the new squamous epithelium were performed 1 month later and then every 4 months once Barrett’s esophagus was considered ablated.
Histopathological assessment
All specimens were reviewed by the same experienced gastrointestinal pathologist (G.M.). Histological samples were fixed in 10% phosphate buffered formalin, embedded in paraffin blocks, and stained with hematoxylin-eosin. According to the Paris classification [20], the samples were classified as metaplastic epithelium, negative for intraepithelial neoplasia, low- or high-grade intraepithelial neoplasia, and intramucosal or invasive carcinoma. In the presence of intramucosal carcinoma, three additional subclasses are described: m1 (intraepithelial), m2 (lamina propria is affected), and m3 (muscularis mucosae is affected). The invasive carcinoma is also divided into three categories: Sm1, Sm2, and Sm3 (the cutoff value to distinguish these categories is 500 μm).
A p value < 0.05 was considered significant for the statistical calculations. The analysis of categorical variables was performed by means of the chi-square test with Yates correction, relative risk with a 95% confidence interval, and Fisher’s exact test. Means were compared using Student’s t-test. The data were processed and analyzed by means of Epi Info version 6.04 and PEPI version 3.0 statistical software.
This study was approved by the Research Ethics Board of our institution, and all patients provided written consent to undergo local therapy after receiving extensive information.
Results
During a mean follow-up of 31.6 (0–83) months, 41 patients were submitted to 63 (mean number:1.5) sessions of EMR (Table 1). Procedure-related complications occurred in 9 (14.3%) patients, consisting of 8 cases of bleeding, which were successfully managed by adrenaline injection and clipping in all cases. There were two perforations, one of them associated with a bleeding episode, but surgery was not required. Only one patient developed symptomatic esophageal stricture, and he was treated by bougienage. Three (7.3%) patients died, but only one as a consequence of the esophageal cancer 24 months after the first EMR and 7 months after the cancer recurrence. Two other patients died because of cardiovascular diseases, with no recurrences up to the conclusion of the study.
Ten (24.4%) patients developed 11 episodes of Barrett’s recurrence after a mean follow-up of 49.6 (3–193) weeks. All of these patients underwent repeat EMR. From this same group, 5 patients (12.2%) developed recurrent (4 cases) or metachronous carcinomas (2 cases) after a mean follow-up of 34.5 (3–72) weeks. Barrett’s esophagus and cancer recurred twice in one patient. All of these patients, except the one referred to surgery, underwent repeat EMR, and three of them received adjuvant therapy with argon plasma coagulation (2 cases) or chemoradiotherapy (1 case). Overall, in an intention-to-treat manner, 31 (75.6%) patients had the metaplastic epithelium completely replaced by squamous epithelium. Including those patients submitted to a repeat EMR after recurrence of metaplastic epithelium or carcinoma, the success rate of the endoscopic approach was 90% (37/41).
Adjuvant therapy was given to 7 patients. Six (14.6%) patients received argon plasma coagulation (4 mucosal cancers and 2 HGD), and two (4.9%) patients with mucosal cancer were submitted to chemoradiotherapy, one of them after argon plasma coagulation.
With regard to the agreement in the histopathological assessment between the pre-EMR biopsies and the EMR specimens, cancer was confirmed in 17 (73.9%) of 23 cases. Another 2 cases were diagnosed as HGD and 4 cases as low-grade dysplasia (LGD) (Table 2). Analyzing adenocarcinoma and multifocal HGD as a single malignant disease, the pre-EMR biopsies diagnosed as cancer overstaged the tumor in 4 (17.4%) of 23 cases classified as LGD after EMR, and in 10 (55.5%) of 18 cases with HGD, classified as LGD (8 cases) and metaplastic epithelium (2 cases) after EMR. Overall, EMR changed the diagnosis and confirmed the presence of lesions of lower neoplastic risk in 14 (34%) patients.
Endoscopic mucosal resection detected cancer in 21 (51.2%) cases. In these patients, the diagnoses obtained by endoscopic biopsies prior to mucosectomy were mucosal cancer (17 cases, 12 of them with a visible lesion) and HGD (4 cases, no visible lesions). The histopathology revealed submucosal involvement in 8 patients (6 cases Sm1 and 2 cases Sm2) previously diagnosed as mucosal cancers by pre-EMR biopsies.
With regard to the EUS evaluation, 21 (91.3%) of 23 cases were correctly classified as mucosal tumors, except for one patient diagnosed as a T2 cancer, in whom the EMR specimen revealed a T1sm2 tumor, and another patient whose tumor was classified as T1N1 cancer, in whom EMR detected only LGD and the lymph node was not punctured. Thus, once cancer was confirmed after EMR, it was overstaged by EUS in 5.9% of the cases. In contrast, when EMR did not confirm the cancer, EUS overstaged the lesions in 16.6% of the cases.
In order to find some predictive factors for recurrent or metachronous carcinomas after EMR, we analyzed some demographic, endoscopic, ultrasonographic, and histopathological aspects. However, no statistical difference was detected (Table 3).
Discussion
Our study presents one of the largest series dealing with circumferential EMR in Barrett’s esophagus related high-grade dysplasia or mucosal cancer. The experience with EMR for neoplastic lesions in BE has increased considerably in the last few years. In the largest series to date, Vieth et al. (22), analyzing 711 samples from 295 patients with superficial neoplasia arising in Barrett’s esophagus submitted to EMR, showed that most of the carcinomas were well-differentiated (72%) and many (93%) were confined to the mucosa. Besides, infiltration of blood and lymphatic vessels was rare. Complete resection was possible in 75% of the patients, 27% during the first attempt and 48% after repeated endoscopic resections. In our experience, esophageal cancer was confined to the mucosal layer in 61.9% (13/21) of the cases, and additional procedures were necessary in 53.7% to enable a complete resection.
Although very promising, to date EMR is restricted to removal of focal lesions, and not long circumferential segments of Barrett’s esophagus. This might seem a logical extension of the use of EMR. Removing not only focal lesions but also the remainder of the metaplastic epithelium might give greater assurance that no neoplasia or columnar mucosa remained, and it might maximize the histological assessment. Reinforcing these advantages, it should be emphasized that EMR avoids operative mortality, which can reach up to 7% in referral centers and up to 20% where the procedure is infrequently performed [5, 6], and enables retained esophageal function.
Following the first case report by Satodate et al. [16] on complete mucosal resection of a BE with early cancer, Seewald et al. [19] performed circumferential EMR in 12 patients with multifocal high-grade intraepithelial neoplasia or mucosal cancer. A polypectomy snare without a cap or submucosal injection was used. The entire BE (median length 5 cm) was completely removed in a median number of 2.5 (1–5) sessions. Bleeding occurred in 4 of 31 (12.9%) sessions, and two (16.6%) patients required esophageal dilation. During a median follow-up of 9 months, no recurrence of Barrett’s esophagus or malignancy was observed.
Our results are in line with those reported by Seewald et al. [19]. The mean size of the Barrett’s epithelium was the same. However, we have already treated a greater number of patients, and our mean follow-up is longer, almost 32 months. In our experience, minor bleeding occurred in 19% of the patients, and 2 perforations (4.8%) were managed clinically. Only one (2.4%) patient developed symptomatic stricture, and the stricture was successfully treated by bougienage. In an intention-to-treat manner, the success rate of the EMR was 75%, reaching 90% when patients submitted to a repeat EMR after recurrence of metaplastic epithelium or carcinoma were included. Metaplastic epithelium and neoplasia recurred in 10 (24%) and 5 (12%) cases, respectively. All of these patients were treated with repeat endoscopic resection. Our recurrence of neoplasia was greater than that reported by Seewald et al. [19], but it is crucial to compare the follow-up periods, almost 3 years in our study and only 9 months in the study of the German group. Furthermore, a review of 161 cases submitted to EMR with mean follow-up of 16 months reported a recurrence rate of 0%–15.8%. In addition to its efficacy, EMR is relatively safe, with significant bleeding and perforation being reported in 0%–14% and 1.8% of procedures, respectively [2, 12]. Besides, our incidence of esophageal stricture was lower, perhaps an advantage of injection of saline into the submucosa, which was not done by Seewald et al. [19], or submitting patients to a repeat EMR session, when necessary, one month after the first. Unfortunately, it is difficult to analyze in detail this finding because the published experience is still too small.
One of the most significant drawbacks of using EMR as a curative treatment is the reliability of preprocedure assessment of the depth of invasion of the neoplasia, as well as the recognition of the lymph node involvement. Although there is some disagreement between the EUS staging and the histopathological assessment of EMR specimens, EUS has been shown to be superior to computed tomography or magnetic resonance imaging for preoperative staging in patients with HGD and cancer [17]. By delineating the tumor infiltration and the lymph node staging, EUS is useful in identifying candidates for endoscopic management. Its accuracy in assessing the depth of infiltration is 80%–85% for T1 tumors [3, 7]. Both overstaging and understaging occur in up to 20% of cases [2, 10, 12, 18]. Among our cases, once cancer was confirmed after EMR, the incidence of EUS overstaging was 5.9%. In contrast, when EMR did not confirm the presence of cancer, EUS overstaging was 16.6%. There were no cases of understaging. Most of mucosal tumors (91.3%) were correctly classified, except for one diagnosed as a T2 cancer, revealed as a T1sm2 tumor after EMR, and another case classified as T1N1, in which EMR detected only LGD and the lymph node was not punctured.
In relation to the EUS staging and the depth of infiltration, in the largest study evaluating the value of EUS with a 20 MHz miniprobe in early esophageal carcinoma and comparing the results with histological findings after EMR, only 2 of 14 patients with submucosal tumor invasion diagnosed after EMR had been correctly diagnosed with EUS beforehand. This finding might explain our 8 patients previously diagnosed with mucosal cancers, in whom the histopathology revealed a submucosal involvement. In addition, sensitivity for mucosal and submucosal tumors was, respectively, 91% and 48%. The EUS understaging and overstaging rates for early esophageal cancer were 13.8% and 6.4%, respectively. Eendoscopic ultrasound overstaging for mucosal cancers was 8.8%. For submucosal cancers, however, EUS understaging was 52% [8]. Our results, even with a lower-frequency radial probe, are similar than those reported by May et al. [8]. With specific reference to lymphadenopathy, quite often small lymph nodes are seen in BE irrespective of the presence of neoplasia, and it is not clear which of these should be aspirated before EMR [21]. For this reason, we did not perform aspiration in the single patient harboring a lymph node.
Indeed, distinguishing among HGD, intramucosal adenocarcinoma, or invasive adenocarcinoma based exclusively on endoscopic biopsies is difficult and subject to interobserver variability [13, 15]. In the experience of Mino-Kenudson et al. [10], EUS correctly reported an intramucosal or submucosal lesion in 70% of the cases. The biopsy diagnosis corresponded to the EMR diagnosis in 63% of the cases. The biopsy resulted in understaging and overstaging in, respectively, 21% and 16% of the cases. These data demonstrate that EMR offers improved diagnosis and staging as compared with endoscopic biopsies and EUS. This is a significant advantage as changes in grade and stage may have a significant impact on patient outcome and enable better clinical decision making [1]. In our experience, cancer was confirmed after EMR in 73.9% of those patients referred to endoscopic treatment. The pre-EMR biopsies diagnosed as cancer overstaged the tumor in 17.4% of the cases. Overall, EMR changed the diagnosis and confirmed the presence of lesions of lower neoplastic risk in 34% of the patients.
Up to now, randomized controlled trials comparing circumferential EMR with surgery or other ablative endoscopic techniques, as well as studies of EMR with long-term results are lacking. The improvement of diagnostic methods, especially EUS, will allow careful selection of patients, minimizing the risk of undertreatment. Technical improvement of the devices and the possibility of avoiding significant strictures after EMR will increase its use.
References
Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA (2001) Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastrointest Endosc 54: 682–688
Ell C, May A, Gossner L, Pech O, Gunter E, Mayer G, Henrich R, Vieth M, Muller H, Seitz G, Stolte M (2000) Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology 118: 670–677
Fleischer D (2000) Endoscopic mucosal resection: (not) made in the USA (so commonly): A dissection of the definition, technique, use, and controversies. Gastrointest Endosc 52: 440–444
Giovannini M, Bories E, Pesenti C, Moutardier V, Monges G, Danisi C, Lelong B, Delpero JR (2004) Circumferential endoscopic mucosal resection in Barrett’s esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy 36: 782–787
Heitmiller RF, Redmond M, Hamilton SR (1996) Barrett’s esophagus with high grade dysplasia: An indication for prophylactic esophagectomy. Ann Surg 224: 66–71
Holscher AH, Bollschweiler E, Schroder W, Gutschow C, Siewert J (1997) Prognostic differences between early squamous-cell and adenocarcinoma of the esophagus. Dis Esophagus 10:179–184
Lightdale CJ (1999) Esophageal cancer. Am J Gastroenterol 94:20–29
May A, Gunter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C (2004) Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: A comparative, prospective, and blinded trial. Gut 53:634–640
May A, Gossner L, Pech O, Muller H, Vieth M, Stolte M, Ell C (2002) Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus: Curative treatment using local endoscopic treatment techniques. Endoscopy 34: 604–610
Mino-Kenudson M, Brugge WR, Puricelli WP, Nakatsuka LN, Nishioka NS, Zukerberg LR, Misdraji J, Lauwers GY (2005) Management of superficial Barrett’s epithelium-related neoplasms by endoscopic mucosal resection: Clinicopathologic analysis of 27 cases. Am J Surg Pathol 29: 680–686
Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Theisen J, Peters JH, Kiyabu M (1999) Occult esophageal adenocarcinoma: Extent of disease and implications for effective therapy. Ann Surg 230: 433–438
Nijhawan PK, Wang KK (2000) Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 52: 328–332
Ormsby AH, Petras RE, Henricks WH, Rice TW, Rybicki LA, Richter JE, Goldblum JR (2002) Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut 51: 671–676
Pellegrini CA, Pohl D (2000) High-grade dysplasia in Barrett’s esophagus: Surveillance or operation? J Gastrointest Surg 4: 131–134
Sampliner RE (2002) Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 97: 1888–1895
Satodate H, Inoue H, Yoshida T, Usui S, Iwashita M, Fukami N, Shiokawa A, Kudo SE (2003) Circumferential EMR of carcinoma arising in Barrett’s esophagus: Case report. Gastrointest Endosc 58: 288–292
Savoy AD, Wallace MB (2005) EUS in the management of the patient with dysplasia in Barrett’s esophagus. J Clin Gastroenterol 39: 263–267
Scotiniotis IA, Kochman ML, Lewis JD, Furth EE, Rosato EF, Ginsberg GG (2001) Accuracy of EUS in the evaluation of Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 54: 689–696
Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S, Soehendra N (2003) Circumferential EMR and complete removal of Barrett’s epithelium: A new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 57: 854–859
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon (2003). Gastrointest Endosc 58: S3–S43
Vieth M, Rosch T (2006) Endoscopic mucosal resection and the risk of lymph-node metastases: Indications revisited? Endoscopy 38: 175–179
Vieth M, Ell C, Gossner L, May A, Stolte M (2004) Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 36: 776–781
Weston AP, Badr AS, Hassanein RS (1999) Prospective multivariate analysis of clinical, endoscopic, and histological factors predictive of the development of Barrett’s multifocal high-grade dysplasia or adenocarcinoma. Am J Gastroenterol 94: 3413–3419
Acknowledgments
Doctor Lopes is financed by grants from CNPq-Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopes, C.V., Hela, M., Pesenti, C. et al. Circumferential endoscopic resection of Barrett’s esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc 21, 820–824 (2007). https://doi.org/10.1007/s00464-006-9187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9187-3