Abstract
Background
Laparoscopic Nissen fundoplication (LNF) is the preferred operation for the control of gastroesophageal reflux disease (GERD). The use of a full fundoplication for patients with esophageal dysmotility is controversial. Although LNF is known to be superior to a partial wrap for patients with weak peristalsis, its efficacy for patients with severe dysmotility is unknown. We hypothesized that LNF is also acceptable for patients with severe esophageal dysmotility.
Methods
A multicenter retrospective review of consecutive patients with severe esophageal dysmotility who underwent an LNF was performed. Severe dysmotility was defined by manometry showing an esophageal amplitude of 30 mmHg or less and/or 70% or more nonperistaltic esophageal body contractions.
Results
In this study, 48 patients with severe esophageal dysmotility underwent LNF. All the patients presented with symptoms of GERD, and 19 (39%) had preoperative dysphagia. A total of 10 patients had impaired esophageal body contractions, whereas 32 patients had an abnormal esophageal amplitude, and 6 patients had both. The average abnormal esophageal amplitude was 24.9 ± 5.2 mmHg (range, 6.0–30 mmHg). The mean percentage of nonperistaltic esophageal body contractions was 79.4% ± 8.3% (range, 70–100%). There were no intraoperative complications and no conversions. Postoperatively, early dysphagia occurred in 35 patients (73%). Five patients were treated with esophageal dilation, which was successful in three cases. One patient required a reoperative fundoplication. Overall, persistent dysphagia was found in two patients (4.2%), including one patient with severe preoperative dysphagia, which improved postoperatively. Abnormal peristalsis and/or distal amplitude improved postoperatively in 12 (80%) of retested patients. There were no cases of Barrett’s progression to dysplasia or carcinoma. During an average follow-up period of 25.4 months (range, 1–46 months), eight patients (16%) were receiving antireflux medications, with six of these showing normal esophageal pH study results.
Conclusion
The LNF procedure provides low rates of reflux recurrence with little long-term postoperative dysphagia experienced by patients with severely disordered esophageal peristalsis. Effective fundoplication improved esophageal motility for most of the patients. A 360° fundoplication should not be contraindicated for patients with severe esophageal dysmotility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastroesophageal reflux disease (GERD) is a common condition troubling nearly 40% of the population [1, 22, 31, 39]. Since 1955, when Nissen performed the first fundoplication for GERD, antireflux surgery has gained popularity, and many investigators have shown it to be very effective in treating complicated GERD [6, 20, 23, 27, 35]. In fact, over the past 10 years, laparoscopic Nissen fundoplication (LNF) has become the preferred surgical technique [2, 17, 20, 28, 32, 35]. A success rate greater than 90% coupled with very low morbidity and mortality figures has resulted in popularization of this technique [2, 7, 12, 21, 25, 36].
Patients with GERD and a nonspecific esophageal motility disorder present a specific challenge. In the past, many experts thought that fundoplication should be tailored to match the strength of the esophageal “pump.” A partial (180–240°) fundoplication was recommended for patients with low distal esophageal amplitudes and a high proportion of simultaneous or nonperistaltic esophageal contractions seen on preoperative esophageal manometry [14, 19, 29, 40]. Mounting clinical evidence, however, has shown that partial fundoplication is not as reliable an antireflux barrier as total fundoplication [10, 13, 15, 30].
On other hand, the fears that improved control of reflux after a full fundoplication would be achieved at the price of higher dysphagia rates [8] have not been substantiated [6, 24, 28, 30]. In fact, Patti et al. [30] has recently concluded that LNF is superior to partial wrap for patients with weak peristalsis (distal esophageal amplitude < 40 mmHg). However, the efficacy and morbidity of the 360° wrap in patients with severe dysmotility is unknown. We hypothesized that LNF is preferable even for patients with severe esophageal dysmotility.
Patients and methods
A multi-institutional review of patients with severe esophageal dysmotility undergoing laparoscopic Nissen Fundoplication was performed. Patients with a history of antireflux surgery were excluded. This analysis included consecutive patients with severe esophageal dysmotility, defined as manometry showing distal esophageal contractions with amplitudes of 30 mmHg or less and/or 70% or more dropped or simultaneous esophageal body contractions. Inpatient and outpatient charts were reviewed, and all data were collected and stored using Excel (Microsoft, Seattle, WA, USA). The data are expressed as average ± standard deviation.
Preoperative evaluation
Patients referred for antireflux surgery underwent a thorough history and physical examination. Flexible upper endoscopy (esophagogastroduodenoscopy), barium esophagram, and esophageal manometry were performed for each patient. In addition, 24-h pH monitoring was performed at the discretion of the attending surgeon. This evaluation was designed to confirm the diagnosis of acid reflux, define the anatomy, rule out additional pathology, and evaluate esophageal motility.
Operative technique
Laparoscopic Nissen fundoplication was performed in standard fashion using five upper abdominal ports. The key features of the operation (with minor variations between the three centers) included a complete dissection of the diaphragmatic crura, complete mobilization of the gastroesophageal junction and distal esophagus to allow 3 to 5 cm of intraabdominal esophagus, complete division of short gastric vessels, and creation of a generous retroesophageal window. The right and left crura were reapproximated using interrupted nonabsorbable sutures. Crural closure was calibrated visually or with a 56- to 60-Fr esophageal bougie. Finally, a short (2–3 cm), loose, 360° fundoplication was performed and secured with interrupted nonabsorbable sutures.
Postoperative care and follow-up evaluation
Patients were allowed to begin drinking clear liquids on the evening of surgery or in the morning of the first postoperative day. The diets were subsequently liberalized. The patients were instructed to avoid meat, bread, and carbonated beverages for the first 2 to 3 weeks after surgery. The patients were seen by the operating surgeon at about 2 and 6 weeks postoperatively, and then as necessary. Postoperative esophageal physiologic testing (24-h esophageal pH and manometry) was performed for select patients. The average duration of the available follow-up period was 25.4 months (range, 1–46 months).
Postoperative dysphagia assessment
Postoperative dysphagia was interpreted to be mild, moderate, or severe according to the type or consistency of food swallowed and the frequency of the dysphagia episodes, as previously described [25] (Table 1). Symptoms resulting from solid foods known to cause dysphagia (i.e., meat and bread products) were distinguished from those associated with other solids in our analysis. All liquids were considered together. Dysphagia was considered to be frequent if it occurred at least once a week. It was considered to be “early” if it occurred or resolved within 8 weeks postoperatively. Dysphagia that persisted longer than 8 weeks was considered to be “late.”
Results
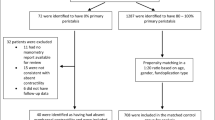
From August of 1998 to July of 2005, 22 women and 26 men with an average age of 54 years underwent an LNF. All the patients presented with primarily “typical” GERD symptoms (Table 2), and 19 patients (39%) had preoperative dysphagia. Four of seven patients with a benign stricture underwent an esophageal dilation preoperatively. Severe esophagitis (grades 3 and 4) was documented preoperatively in 25 patients (52.1%). Barrett’s esophagus was found in 19 patients (39.6%). According to preoperative 24-h esophageal pH testing, the average percentage of esophageal acid exposure (pH < 4) time was 17.9% (range, 1.7– 68%) for the 41 patients tested. The average DeMeester score was 76 (range, 12–341).
All the patients had motility testing before surgery. The average lower esophageal sphincter resting pressure was 9.9 mmHg (1.1–24.6 mmHg). A total of 10 patients had dropped or simultaneous contractions for more than 70% of swallows, whereas 32 patients had abnormal amplitudes of contraction of distal esophagus, and 6 patients had both. The average abnormal esophageal amplitude was 24.9 ± 5.2 mmHg (range, 6–30 mmHg). The mean proportion of nonperistaltic esophageal body contractions was 79.4% ± 8.3% (range, 70–100%).
Laparoscopic Nissen fundoplication was performed for all the patients. Six patients (12.5%) underwent concomitant esophageal lengthening (n = 2) or gastric emptying (n = 4) procedures. There were no conversions to an open procedure. There were no major perioperative complications or mortalities. The average length of hospital stay was 1.6 ± 0.4 days (range, 1–4 days).
Initially, dysphagia occurred in 35 patients (72.9%), including severe dysphagia in 4 patients (8.3%) (Fig. 1). This early dysphagia resolved spontaneously by 8 weeks for 26 patients (74.3%). One patient required a dilation at 2 weeks postoperatively due to an episode of food impaction. Dysphagia remained unresolved after 8 weeks postoperatively for only eight patients (16%), including four patients with preoperative dysphagia. Late spontaneous resolution of dysphagia was noted in three patients at 3 to 12 months postoperatively. The remaining five patients underwent an esophageal bougie dilation at 2 to 11 months postoperatively, with resolution of symptoms in three patients. One patient required reoperative partial fundoplication for dysphagia 6 months postoperatively, and experienced minor improvement in symptoms. Persistent dysphagia was noted in two patients (4.2%). One of these patients had preexisting severe dysphagia that actually had improved since surgery.
A total of 15 patients had postoperative esophageal motility testing. Abnormal peristalsis and/or contraction amplitude improved postoperatively in 12 (80%) of retested patients. No patients had worsening of their esophageal function. During an average follow-up period of 25.4 months (range, 1–46 months), eight patients (16.7%) were receiving daily antireflux medications for vague dyspeptic symptoms. Six (12.7%) of these patients, however, had documented normal esophageal pH studies.
Discussion
Esophageal dysmotility is a known sequela of long-standing reflux disease. In addition to intrinsic disorders of esophageal motility, both impairments of esophageal peristalsis and insufficient distal amplitudes may stem from chronic esophageal acid exposure [10]. Moreover, the distal dysmotile segment can then potentiate the injury and lead to even greater esophageal acid exposure attributable to ineffective esophageal clearance of the refluxed acid [13, 28]. Because fundoplication restores cardioesophageal competence and increases the high pressure zone at the gastroesophageal junction, the resistance to flow of food bolus through the impaired distal esophagus has long been considered a risk factor for severe postoperative dysphagia. In the past, many experts thought that fundoplication should be tailored to match the strength of the esophageal “pump.” A partial (180–240°) fundoplication was recommended for patients with low distal esophageal amplitudes and a high proportion of simultaneous or nonperistaltic esophageal contractions seen on preoperative esophageal manometry [14, 29, 40].
Mounting clinical evidence, however, has shown that partial fundoplication is not as reliable or durable an antireflux barrier as total fundoplication [6, 28, 30]. In the recent review of the “tailored approach” experience at UCSF, Patti et al. [30] reported that 19% of the patients who underwent a partial fundoplication had objective evidence of symptomatic reflux. Laparoscopic Nissen fundoplication, on the other hand, resulted in a much lower (4%) symptomatic failure rate. Similarly, Horvath et al. [13] reported that although a laparoscopic partial (Toupet) fundoplication was well tolerated, the long-term results showed failure rates of 20% and abnormal pH scores in 59% of patients. In addition, 64% had resumed oral antacid therapy. These findings prompted the authors to conclude that partial wraps were inadequate for patients with severe GERD [13]. Bell et al. [5] reported a 50% symptomatic failure rate 3 years after a partial fundoplication. Thus, it appears that a partial wrap may not be adequate to provide a reliable barrier to gastroesophageal reflux in most patients. Although we identified eight patients (16.7%) receiving daily antireflux medications postoperatively, six of them had no evidence of esophageal acid exposure on postoperative pH studies. It appears that Nissen fundoplication was a reliable antireflux procedure even in this difficult group of patients with severe reflux.
We have essentially abandoned a tailored approach to fundoplication. In fact, most preoperative dysphagia improves after an effective antireflux procedure because elimination of acid exposure improves esophageal motility and esophageal clearance [4, 6, 24, 28, 34]. The evidence from this study supports the conclusion that GERD patients with defective esophageal peristalsis can undergo a well-constructed total fundoplication without a significantly increased risk of dysphagia. In fact, other investigators have shown that postoperative dysphagia rates are not affected by preoperative motility [10, 11, 28, 41]. Although Patti et al. [30] reported that patients undergoing a postoperative esophageal dilation to relieve postoperative dysphagia were more likely to have had a full wrap (1.3% vs 3.6%; p < 0.05), no persistent dysphagia was noted in either the full or the partial fundoplication group.
In the current series, we found that five patients (10.1%) required postoperative dilations. These were single-session well-tolerated procedures with complete symptomatic improvement in 80% of the patients. Although the overall persistent postoperative dysphagia occurred in two patients (4.2%), dysphagia developed in only one of these patients after the surgery. In other words, 18 (94.7%) of 19 patients experienced relief of preoperative dysphagia symptoms.
Overall, it appears that a full fundoplication is an acceptable, and perhaps preferred, procedure even for patients with severe dysmotility, including those with preoperative dysphagia, because it reduces the rate of surgical failure with little postoperative dysphagia. However, the “gold standard” determination cannot be given for the Nissen fundoplication in this patient population until the effects of a full wrap over the long term (5–10 years) become known.
Patients with Barrett’s esophagus present an additional challenge. The prevalence of esophageal dysmotility in this patient subgroup is disproportionably high [27]. In the current series, nearly 40% of the patients had Barrett’s esophagus preoperatively. It is likely that severe and long-standing reflux disease that caused metaplastic changes also contributed to the development of motility problems as well. Because surgical correction of reflux is the most effective means of halting the progression to dysplasia [18, 26, 27] and can lead to a reversal of metaplastic or dysplastic changes in some patients [27], we believe patients with Barrett’s esophagus should be provided with the benefits of the most effective reflux barrier. As a result, a full fundoplication may be of particular importance for patients with Barrett’s esophagus even in the presence of esophageal dysmotility. During an average follow-up period longer than 2 years, none of our patients progressed to dysplasia or adenocarcinoma.
Defining esophageal dysmotility has not been consistent in the surgical literature. Most surgeons would agree that a distal esophageal amplitude exceeding 40 mmHg and at least 70% peristaltic contraction of the esophageal body indicate normal motility. Patti et al. [30] defined all patients with distal amplitudes below 40 mmHg as defective. Similar criteria also have been used by other investigators [4, 11, 16, 28, 41]. Although amplitudes of contraction in the midesophagus are not routinely used to define dysmotility, these parameters may be useful in identifying so-called secondary or reflux-induced disordered motility. Furthermore, the proportion of nonperistaltic contraction considered pathologic has varied in the literature as well. Most authors have considered patients with 30% to 60% simultaneous or nonpropagating contractions to have defective esophageal peristalsis [3, 9–11, 13, 27, 41]. We have used the more stringent criteria (distal amplitudes less than 30 mmHg and at least 70% nonperistaltic esophageal body contractions) to define severe esophageal dysmotility.
Multichannel intraluminal impedance (MII) recently has been introduced for the evaluation of esophageal function and reflux disease [33]. This technology provides data on intraluminal pressure changes and food bolus movement throughout the esophagus. Swallows on MII are classified as complete or incomplete. The study is normal if 80% of liquid swallows and at least 70% of viscous swallows show a complete bolus transit [38]. Advocates of MII have touted it as more reflective of esophageal dysmotility than the abnormal traditional manometry indices [37]. Although not widely used today, this form of esophageal testing may bring a shift in definitions of esophageal dysmotility, with significant implications for future preoperative testing. As MII technology is adopted, an additional study of full fundoplication for patients with esophageal dysmotility using this testing method would be appropriate.
Conclusion
Laparoscopic Nissen fundoplication is a reliable antireflux barrier even in patients with severe esophageal dysmotility. This is achieved without a significant rate of postoperative dysphagia. Furthermore, a 360° fundoplication resulted in improved postoperative motility for 80% of patients, with resolution of preoperative dysphagia for 95% of the patients in this series. A full fundoplication may be of particular importance for patients with Barrett’s esophagus to prevent disease progression to dysplasia or adenocarcinoma. Impedanace manometry may become an important method for evaluating preoperative esophageal motility. Overall, severely abnormal distal esophageal amplitudes or a high proportion of nonperistaltic esophageal contractions should not be viewed as a contraindication to a Nissen fundoplication. Prospective trials, however, are needed to confirm the findings of this study, to establish the degree of esophageal dysmotility that prohibits a full fundoplication, and to examine the long-term results in this patient population.
References
Laparoscopic antireflux surgery for gastroesophageal reflux disease (GERD) (1997) Results of a Consensus Development Conference. Held at the Fourth International Congress of the European Association for Endoscopic Surgery (E.A.E.S.), Trondheim, Norway, 21–24 June 1996. Surg Endosc 11: 413–426
Anvari M, Allen C (2003) Five-year comprehensive outcomes evaluation in 181 patients after laparoscopic Nissen fundoplication. J Am Coll Surg 196: 51–57; discussion 57–58, author reply 58–59
Baigrie RJ, Watson DI, Myers JC, Jamieson GG (1997) Outcome of laparoscopic Nissen fundoplication in patients with disordered preoperative peristalsis. Gut 40: 381–385
Beckingham IJ, Cariem AK, Bornman PC, Callanan MD, Louw JA (1998) Oesophageal dysmotility is not associated with poor outcome after laparoscopic Nissen fundoplication. Br J Surg 85: 1290–1293
Bell RC, Hanna P, Mills MR, Bowrey D (1999) Patterns of success and failure with laparoscopic Toupet fundoplication. Surg Endosc 13: 1189–1194
Biertho L, Sebajang H, Anvari M (2006) Effects of laparoscopic Nissen fundoplication on esophageal motility: long-term results. Surg Endosc 20: 619–623
Catarci M, Gentileschi P, Papi C, Carrara A, Marrese R, Gaspari AL, Grassi GB (2004) Evidence-based appraisal of antireflux fundoplication. Ann Surg 239: 325–337
DeMeester TR, Stein HJ (1992) Minimizing the side effects of antireflux surgery. World J Surg 16: 335–336
Diaz de Liano A, Oteiza F, Ciga MA, Aizcorbe M, Trujillo R, Cobo F (2003) Nonobstructive dysphagia and recovery of motor disorder after antireflux surgery. Am J Surg 185: 103–107
Farrell TM, Archer SB, Galloway KD, Branum GD, Smith CD, Hunter JG (2000) Heartburn is more likely to recur after Toupet fundoplication than Nissen fundoplication. Am Surg 66: 229–236; discussion 236–237
Fibbe C, Layer P, Keller J, Strate U, Emmermann A, Zornig C (2001) Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology 121: 5–14
Granderath FA, Kamolz T, Schweiger UM, Pasiut M, Haas CF, Wykypiel H, Pointner R (2002) Long-term results of laparoscopic antireflux surgery. Surg Endosc 16: 753–757
Horvath KD, Jobe BA, Herron DM, Swanstrom LL (1999) Laparoscopic Toupet fundoplication is an inadequate procedure for patients with severe reflux disease. J Gastrointest Surg 3: 583–591
Hunter JG, Trus TL, Branum GD, Waring JP, Wood WC (1996) A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg 223: 673–685; discussion 685–687
Jobe BA, Wallace J, Hansen PD, Swanstrom LL (1997) Evaluation of laparoscopic Toupet fundoplication as a primary repair for all patients with medically resistant gastroesophageal reflux. Surg Endosc 11: 1080–1083
Kahrilas PJ, Dodds WJ, Hogan WJ, Kern M, Arndorfer RC, Reece A (1986) Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology 91: 897–904
Kamolz T, Granderath PA, Bammer T, Pasiut M, Wykypiel H Jr, Herrmann R, Pointner R (2002) Mid- and long-term quality of life assessments after laparoscopic fundoplication and refundoplication: a single unit review of more than 500 antireflux procedures. Dig Liver Dis 34: 470–476
Katz D, Rothstein R, Schned A, Dunn J, Seaver K, Antonioli D (1998) The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett’s esophagus. Am J Gastroenterol 93: 536–541
Kauer WK, Peters JH, DeMeester TR, Heimbucher J, Ireland AP, Bremner CG (1995) A tailored approach to antireflux surgery. J Thorac Cardiovasc Surg 110: 141–146; discussion 146–147
Lafullarde T, Watson DI, Jamieson GG, Myers JC, Game PA, Devitt PG (2001) Laparoscopic Nissen fundoplication: five-year results and beyond. Arch Surg 136: 180–184
Liu JY, Woloshin S, Laycock WS, Schwartz LM (2002) Late outcomes after laparoscopic surgery for gastroesophageal reflux. Arch Surg 137: 397–401
Locke GR III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ III (1997) Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112: 1448–1456
Mahon D, Rhodes M, Decadt B, Hindmarsh A, Lowndes R, Beckingham I, Koo B, Newcombe RG (2005) Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 92: 695–699
Munitiz V, Ortiz A, Martinez de Haro LF, Molina J, Parrilla P (2004) Ineffective oesophageal motility does not affect the clinical outcome of open Nissen fundoplication. Br J Surg 91: 1010–1014
Novitsky YW, Kercher KW, Callery MP, Czerniach DR, Kelly JJ, Litwin DE (2002) Is the use of a bougie necessary for laparoscopic Nissen fundoplication? Arch Surg 137: 402–406
Oberg S, Wenner J, Johansson J, Walther B, Willen R (2005) Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg 242: 49–54
Oelschlager BK, Barreca M, Chang L, Oleynikov D, Pellegrini CA (2003) Clinical and pathologic response of Barrett’s esophagus to laparoscopic antireflux surgery. Ann Surg 238: 458–464; discussion 464–466
Oleynikov D, Eubanks TR, Oelschlager BK, Pellegrini CA (2002) Total fundoplication is the operation of choice for patients with gastroesophageal reflux and defective peristalsis. Surg Endosc 16: 909–913
Patti MG, De Pinto M, de Bellis M, Arcerito M, Tong J, Wang A, Mulvihill SJ, Way LW (1997) Comparison of laparoscopic total and partial fundoplication for gastroesophageal reflux. J Gastrointest Surg 1: 309–315
Patti MG, Robinson T, Galvani C, Gorodner MV, Fisichella PM, Way LW (2004) Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. J Am Coll Surg 198: 863–869; discussion 869–870
Rattner DW (2000) Measuring improved quality of life after laparoscopic Nissen fundoplication. Surgery 127: 258–263
Ritter DW, Vanderpool D, Westmoreland M (1997) Laparoscopic Nissen fundoplication for gastroesophageal reflux disease. Am J Surg 174: 715–717; discussion 717–718
Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J, Castell D (2004) Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol 99: 1037–1043
Stein HJ, Bremner RM, Jamieson J, DeMeester TR (1992) Effect of Nissen fundoplication on esophageal motor function. Arch Surg 127: 788–791
Terry M, Smith CD, Branum GD, Galloway K, Waring JP, Hunter JG (2001) Outcomes of laparoscopic fundoplication for gastroesophageal reflux disease and paraesophageal hernia. Surg Endosc 15: 691–699
Trus TL, Laycock WS, Waring JP, Branum GD, Hunter JG (1999) Improvement in quality-of-life measures after laparoscopic antireflux surgery. Ann Surg 229: 331–336
Tutuian R, Vela MF, Balaji NS, Wise JL, Murray JA, Peters JH, Shay SS, Castell DO (2003) Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol 1: 174–182
Tutuian R, Vela MF, Shay SS, Castell DO (2003) Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol 37: 206–215
Valle C, Broglia F, Pistorio A, Tinelli C, Perego M (1999) Prevalence and impact of symptoms suggestive of gastroesophageal reflux disease. Dig Dis Sci 44: 1848–1852
Wetscher GJ, Glaser K, Wieschemeyer T, Gadenstaetter M, Prommegger R, Profanter C (1997) Tailored antireflux surgery for gastroesophageal reflux disease: effectiveness and risk of postoperative dysphagia. World J Surg 21: 605–610
Zornig C, Strate U, Fibbe C, Emmermann A, Layer P (2002) Nissen vs Toupet laparoscopic fundoplication. Surg Endosc 16: 758–766
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novitsky, Y.W., Wong, J., Kercher, K.W. et al. Severely disordered esophageal peristalsis is not a contraindication to laparoscopic Nissen fundoplication. Surg Endosc 21, 950–954 (2007). https://doi.org/10.1007/s00464-006-9126-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9126-3