Abstract
Background
When we perform laparoscopic lymph node dissection around the inferior mesenteric artery (IMA), we preserve the left colic artery (LCA) to maintain the blood supply to the proximal sigmoid colon. In this study, we present our laparoscopic D2 and D3 lymph node (LN) dissection technique and evaluate its applicability and safety.
Methods
We performed LN dissection on 23 rectal and lower sigmoid colon cancer cases from April 2002 to December 2004. For D3 LN dissection, the incision to the mesosigmoid extends to just before the root of the IMA, which is exposed with an ultrasonic cutting and coagulating surgical device to avoid bleeding. Then, the arterial wall is exposed with a dissecting electrocautery spatula down to the LCA, at least 2 cm of which is exposed. Adipose tissue surrounding the IMA and inferior mesenteric vein is dissected. For D2 LN dissection, we partially expose the IMA to confirm the location of the LCA.
Results
The mean times taken for D2 and D3 LN dissections were 36.2 and 68.2 min, respectively. Both procedures took longer in male patients. There was a trend for the procedure overall to take less time in female patients. However, D2 dissection took significantly longer in male than female patients (p < 0.05). In women, D3 dissection took significantly longer than D2 (p < 0.05), but this trend was not seen in men. Increased experience among surgeons with this procedure was associated with significantly faster LN dissections in men (p < 0.05), but not in women (p = 0.493). Pearson product moment analysis identified a relationship between body mass index (BMI) and the time taken for D2 LN dissection (r = 0.765), but not D3 LN dissection (r = 0.158).
There was no treatment-related morbidity with this technique.
Conclusions
This method was safe and feasible for all patients in this series, but takes longer to perform in male patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic-assisted colorectal surgery (LAC) has become an accepted surgical modality for neoplasms of the colon and rectum. Since January 1997, we have used LAC for the treatment of benign colorectal tumors and early colon cancer that could not be resected endoscopically or that showed submucosal invasion. As we refined this technique, we began to apply it from January 2000 to the treatment of advanced colorectal cancer, but not bulky advanced rectal cancer (Rs, Ra) or advanced lower rectal cancer (Rb). Initially we took a lateral approach for LAC, in which mobilization of the colon preceded the lymph node (LN) dissection, but since April 2002, we have used a median approach, in which lymph node dissection and vessel dissection precedes the mobilization of the colon.
There are few reports of comparison between Japanese and Caucasians concerning the lengths of the various parts of the colon. From textbooks of anatomy, the mean lengths of the sigmoid colon in Japanese and Caucasians are 45 cm and 40 cm, respectively. The sigmoid colon of Japanese is believed to be longer than that of Caucasians. This anatomic difference has made it possible to preserve the proximal sigmoid colon even during open surgery of rectal and lower sigmoid colon cancer in our department. For left-side colon cancer, it is usual to cut the root of the inferior mesenteric artery (IMA) to perform D3 LN dissection. We preserve the left colic artery (LCA) in both D2 and D3 dissections to maintain the blood supply to the preserved long proximal sigmoid colon.
In this study, we present our laparoscopic D2 and D3 LN dissection technique and evaluate its applicability and safety.
Materials and methods
From April 2002 to August 2004, 24 rectal and lower sigmoid colon cancer patients underwent laparoscopic-assisted high or low anterior resection with D2 or D3 LN dissection in our department (Table 1). We perform a D2 LN dissection around the IMA for early cancer cases with minimal submucosal (sm) invasion and a D3 LN dissection for extensive sm invasion and advanced cases.
Surgical technique
In general, placement of the trocars depends on the shape of the abdomen and the location of the tumor. The laparoscope is inserted thorough a 12-mm trocar placed infraumbilically in most cases. Two trocars are inserted in the right lower quadrant for the operator, and a 5-mm trocar is inserted in the left lateral lower quadrant for the assistant. An additional 12-mm trocar is placed in the left lower quadrant if needed. The patient is placed in a steep Trendelenburg position, the small intestine is pulled cephalad so that the entire operative field is visible, and then lymph node dissection is performed following the mobilization of the sigmoid colon. The assistant holds the mesosigmoid including the pedicle of vessels ventrally and to the left.
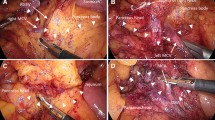
For a D3 dissection, we first incise the right side of the mesocolic peritoneal sheet of the sigmoid colon, anterior to the right iliac artery (Fig. 1a). The incision continues cephalad until just before the origin of the IMA with a dissecting electrocautery spatula (Fig. 1b). The root of the IMA is dissected with an ultrasonic cutting and coagulating surgical device (LCS) to avoid bleeding from the right side to the cephalad side over the IMA origin, thereby avoiding any injury to the duodenum (Fig. 1c). The superior hypogastric nerve should be preserved during the procedure. Then the artery wall is exposed with a dissecting electrocautery spatula down to the root of the LCA (Fig. 1d). During this procedure, cauterization is used only when entering into the dissection layer, cutting the nerve plexus around vessel, and cutting small vessels to avoid bleeding. Otherwise we do not use cauterization, instead just using this device as a spatula. At least 2 m of the LCA should be exposed where the inferior mesenteric vein (IMV) can be recognized for D3 dissection (Fig. 1e, 1f). The sigmoidal artery arising from the LCA is usually clipped and cut, and then the IMV is clipped and cut at the cranial side of the left colic artery (Fig. 1g). Finally, adipose tissue including LNs in the area surrounding the IMA, IMV, and LCA is dissected with LCS (Fig. 1h). The root of the superior rectal artery is clipped and cut.
A The first incision of the mesentery of the sigmoid colon. RIA: right common iliac artery. B The incision continues to the root of the IMA with electric cautery. C The root of the IMA is dissected with LCS to avoid bleeding. D The lymph nodes around the IMA are dissected to expose the IMA wall using a dissecting electrocautery spatula. Note that cauterization should be minimized. E The bifurcation of the LCA and SRA is exposed. F The LCA is exposed until the IMV can be recognized. G The IMV is clipped and cut. H Adipose tissue surrounded by the IMA, LCA, and IMV is dissected with LCS. I The mesosigmoid is mobilized posteriorly in a medial-to-lateral fashion to expose the left ureter and gonadal vessels.
For D2 LN dissection, we do not expose the root of the IMA, but the IMA is partially exposed to confirm the root of the left colic artery and the root of the superior rectal artery.
After LN dissection, the mesosigmoid is mobilized posteriorly in a medial-to-lateral fashion, maintaining the layer on the left ureter and gonadal vessels (Fig. 1i). Then, a leaf of gauze is placed on the left ureter and the gonadal vessels to maintain the correct layer during dissection of the lateral attachment of the sigmoid colon.
Anastomosis was usually done by double stapling with endoscopic linear stapler and dircular stapler. The blood supply to the oral side of the sigmoid colon is recognized by observing the macroscopic pulsating of the small artery.
Statistical analysis
Chi-square test was used to compare the time taken for LN dissection between D2, D3, men, and women. Cochran-Mantel-Haenszel analysis was used to compare the time taken for LN dissection and clinical experience. Pearson product moment analysis was used to examine the relation between the time taken for LN dissection and body mass index (BMI).
Results
All patients underwent LN dissection around the IMA by laparoscope. However, one (case 8) underwent open surgery after the laparoscopic LN dissection because of massive subcutaneous emphysema. The mean time from the first incision on the mesentery to completion was significantly longer for D3 LN dissection (36.2 min, range 19–94) than for D2 LN dissection (68.2 min, range 37–124; p < 0.05) (Table 2). There was a trend for the procedure overall to take less time in female patients, but this difference was not significant (p = 0.072). However, D2 dissection specifically took significantly longer in male than female patients (p < 0.05). In women, D3 dissection took significantly longer than D2 (p < 0.05), but this trend was not seen in men. Increased experience among surgeons with this procedure was associated with significantly faster LN dissections in men (p < 0.05), but not in women (p = 0.493) (Fig. 2), but when patients were grouped together there was no relationship between experience and time taken for D2 or D3 dissection (Fig. 3). Pearson product moment analysis identified a relationship between BMI and the time taken for D2 LN dissection (r = 0.765), but not D3 LN dissection (r = 0.158) (Fig. 4).
There was no case of intraoperative or postoperative morbidity related to our LN dissection technique, including no bleeding due to vessel injury (Table 1). One patient had anastomotic failure, due to failure of the circular stapler, not due to ischemia, which was improved with conservative treatment. Two patients had wound infections, one of whom showed ileus 2 months after operation, and there were two cases of diarrhea on the first day after surgery.
Discussion
Here we describe our laparoscopic D2 and D3 LN dissection technique for colorectal cancer in which we strive to preserve the LCA to maintain the blood supply to the preserved long proximal sigmoid colon. The blood supply and lymphatic system of the colon are relatively simple compared to those of the stomach. For gastric cancer, D1 LN dissection is accomplished by cutting the root of the major vessels of the stomach. For D2 dissection, however, adipose tissue including LNs is dissected around major arteries, such as the common hepatic artery, proper hepatic artery, celiac axis, and splenic artery, preserving those arteries. Because of the simplicity of the blood supply and lymphatic system of the colon, the vessels are cut at their root for LN dissection.
We retrospectively analyzed the results of this approach in 23 patients to determine which factors may affect the time taken to complete the procedure, and any associated morbidity or mortality. D2, but not D3, dissection took significantly longer in men, and increased experience among surgeons with this procedure was associated with significantly faster LN dissections in male patients. In addition, increasing BMI was associated with increased time for the procedure. In general, men have more visceral adipose tissue than women, who have more subcutaneous adipose tissue. It is possible that thicker fat in the mesentery in men obscured the root of the LCA during D2 dissections. Therefore, it may be appropriate to select patients with lower BMIs for less experienced surgeons.
The marginal artery of Drummond is occasionally tenuous at the splenic flexure, and is absent in 5% of patients, which is called the Griffiths point [2]. An area 1.5–2.8 cm2 may be devoid of vasa recta [1]. In the West, the splenic flexure is sometimes freed to maintain the length of the proximal colon, when the IMA is cut at its root [6] Even among Japanese, some patients have shorter sigmoid colons. While we do not usually free the splenic flexure, we experience no abnormal tension of the proximal sigmoid colon, because in our Japanese patients we are able to preserve a sufficient length of proximal sigmoid colon. When the proximal sigmoid colon is resected, division of the LCA allows the descending colon to extend into the pelvis with less tension [5]. Therefore, in Caucasians, who have a shorter sigmoid colon, preservation of the LCA may help limit the resection of the sigmoid colon, removing the need for mobilization of the splenic flexure. Moreover, the pulsing of the small artery on the oral side of the sigmoid colon is visible in almost all cases.
This method of LN dissection in colorectal cancer was very safe. There was no postoperative mortality, and no procedure-related morbidity including bleeding complications due to vessel injury. By maintaining the blood supply to the proximal sigmoid colon we expected to prevent anastomotic failure due to ischemia, and consistent with this, we experienced only one anastomotic failure, which was due to failure of the circular stapler.
The LCA is absent in 12% of individuals, in whom the colosigmoid artery performs this function [4, 7]. There can be a large distance between the root of the IMA and that of the LCA in some cases, which can make LN dissection around the IMA difficult until the LCA is recognized. Recent advances in multidetector CT (MD-CT) imaging enable the reconstruction of three-dimensional images of the mesenteric vessels, such as the superior mesenteric artery and vein, IMA, and IMV [3]. We investigated the distance between the origin of the IMA and that of the LCA by MD-CT in 50 consecutive Japanese patients with colorectal cancer. Two of the 50 patients lacked the LCA. Three-dimensional images indicated that the average distance between these two origins is 40.3 mm (25.3–75.0 mm) (data not shown).
Conclusion
In conclusion, our procedure for LN dissection around the IMA for rectal and lower sigmoid colon cancer is safe and was applicable to all patients in this series. Moreover, it may avoid unnecessary resection of the proximal colon or mobilization of the splenic flexure.
References
Baixauli J, Kiran RP, Delaney CP (2003) Investigation and management of ischemic colitis. Cleveland Clin J Med 70: 920–934
Griffiths JD (1956) Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl 19: 241–256
Horton KM, Fishman EK (2000) 3D CT angiography of the celiac and superior mesenteric arteries with multidetector CT data sets: preliminary observations. Abdom Imaging 25: 523–525
Kornblith PL, Boley SJ, Whitehouse BS (1992) Anatomy of the splanchnic circulation. Surg Clin North Am 72: 1–30
Milsom JW, Böhm B (1996) Laparoscopic colorectal surgery. Springer-Verlag, New York, pp 117–194
Milsom JW, Böhm B, Decanini C, Fazio VW (1994) Laparoscopic oncologic proctosigmoidectomy with low colorectal anastomosis in a cadaver model. Surg Endosc 8: 1117–1123
Rosenblum JD, Boyle CM, Schwartz LB (1997) The mesenteric circulation: anatomy and physiology. Surg Clin North Am 77: 289–306
Acknowledgment
This study was supported by Kobayashi Magobe Memorial Medical Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) meeting, Fort Lauderdale, FL, USA, 13–16 April 2005
Rights and permissions
About this article
Cite this article
Kobayashi, M., Okamoto, K., Namikawa, T. et al. Laparoscopic lymph node dissection around the inferior mesenteric artery for cancer in the lower sigmoid colon and rectum. Surg Endosc 20, 563–569 (2006). https://doi.org/10.1007/s00464-005-0160-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0160-3