Abstract
Laparoscopic D3 lymph node dissection for transverse colon cancer is technically demanding because of complicated anatomy. Here, we reviewed the vascular structure of the transverse mesocolon, explored the extent of the base of the transverse mesocolon, and evaluated the feasibility and oncological safety of D3 lymph node dissection. We retrospectively reviewed the clinical records of 42 patients with advanced transverse colon cancer who underwent curative surgery and D3 dissection at Kyushu University Hospital between January 2008 and December 2015. We examined the venous and arterial anatomy of the transverse mesocolon of each resection and compared surgical outcomes between patients who underwent laparoscopic D3 (Lap D3) and open D3 (Open D3) dissection. Patients included two with Stage I, 18 with Stage II, 20 with Stage III, and two with Stage IVA. Thirty-six (85.7%) and six (14.3%) patients underwent Lap D3 or Open D3, respectively. The tumor sizes of the Open D3 and Lap D3 groups were 7.8 and 3.7 cm, respectively (P < 0.001). The Lap D3 group had significantly less blood loss (26 mL vs 272 mL, P = 0.002). The other outcomes of the two groups were not significantly different, including 3-year overall survival (87.7% vs 83.3%, P = 0.385). We observed four patterns of the middle colic artery (MCA) arising from the superior mesenteric artery (SMA), and the frequency of occurrence of a single MCA was 64.3%. The right-middle colic vein (MCV) was present in 92.9% of resections and served as a tributary of the gastrocolic trunk, and 90.5% of the left MCVs drained into the superior mesenteric vein (SMV). The root of the transverse mesocolon was broadly attached to the head of the pancreas and to the surfaces of the SMV and SMA. Laparoscopic D3 lymph node dissection may be tolerated by patients with advanced transverse colon cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
After the introduction of total mesorectal excision [1] for rectal cancers and its subsequent use worldwide, a similar procedure called complete mesocolic excision (CME) for colon cancers was published by Hohenberger et al. [2]. The purpose of these surgical procedures is complete removal of the mesorectum or mesocolon without disrupting the wrapping of the invading tumor and its lymphovascular drainage as well as possible metastatic lymph nodes. These procedures are accomplished through the application of precise dissection according to the embryological plane. CME is often coupled with central vascular ligation (CVL) to achieve sufficient mesocolic resection, which decreases the local recurrence rate and improves survival of patients compared with “standard surgery” performed in Western countries [2, 3]. Sophisticated interpretations of the embryological tissue planes of the mesocolon that are required to perform CME are available for right- and left-side colectomies [4, 5]. In contrast, the extent of the transverse mesocolon (TMC) that contains regional lymph nodes and the detailed vascular anatomy of the transverse colon are not adequately defined. Moreover, most clinical trials that examine the safety and efficiency of laparoscopic surgery for colon cancer exclude transverse colon cancer (TCC), mainly because of the potential difficulty in dividing the middle colic vessels at their roots and in resecting a sufficient area of the TMC [6,7,8].
In Japan, lymph nodes harvested during colorectal cancer resection are categorized along with their locations as follows: (1) pericolic D1 nodes near the bowel wall, (2) intermediate D2 nodes adjacent to the supplying arteries, and (3) main D3 nodes at the origins of the supplying arteries [9]. D2 resection for early colon cancers and D3 resection for advanced colon cancers, coupled with 10-cm longitudinal resection of the bowel away from the tumor on either side, is a principle of colon cancer resection in Japan [10]. The Japanese “10-cm rule” of bowel resection was established through meticulous study of the distribution of metastasized lymph nodes using the clearing method for colonic carcinomas [10,11,12]. Despite the smaller mesentery and the retrieval of fewer lymph nodes, the survival rates of patients with colon cancer who undergo D3 dissection are comparable with those who undergo CME with CVL [13].
In Japan, the procedure is performed according to tumor location as follows: right hemicolectomy of the right-side TCC dividing the ileocolic artery (ICA), the right colic artery (RCA), the right branch of the middle colic artery (MCA); transverse colectomy of the mid-TCC dissecting the middle colic artery (MCA); and partial resection of the transverse and descending colon for the left-side TCC separating the left branch of the MCA and the left colic artery (LCA) [14, 15]. These bowel- and vessel-preserving procedures are beneficial, because they avoid performing a troublesome total colectomy upon the second surgery to remove subsequent colorectal cancers, because patients with colorectal cancer frequently develop metachronous cancers [16, 17].
Although retrospective studies demonstrate the short-term benefits and oncological safety of laparoscopic surgery for TCC [14, 18, 19], the extent of TMC, including vascular anatomy, are not fully addressed by these studies, and the detailed surgical procedures are not presented [20]. To gain a detailed understanding of the relevant surgical anatomy and to improve the safety of sufficient lymph node dissection, we conducted a single-institution retrospective study of the topographic variance of the supplying arteries and draining veins in the TMC. Such knowledge allows the surgeon to determine the extent of the TMC containing the tumor-draining lymphatics and vessels as well as the regional lymph nodes. Here, we describe our laparoscopic procedures for transverse colectomy with D3 lymph node dissection using bidirectional dissection of the TMC and assess the outcomes of patients to indicate the feasibility of this procedure.
Methods
Patients
We reviewed the records of 951 patients who underwent resection of colorectal cancer at the Department of Surgery and Oncology, Kyushu University Hospital. We identified 42 patients with advanced transverse colon cancer who underwent D3 lymph node dissection between January 2008 and October 2015. We conducted a serial review of their records to investigate the origin of the MCA. The surgical records included the numbers and origins of arteries, the veins that traverse the TMC, and the transection points of these vessels. Other clinical and pathological data were obtained from medical records. Staging of the patients was performed according to the 7th TNM classification system [21], whereas regional lymph nodes were classified into pericolic, intermediate, and main groups, according to their location relative to the MCA, following the Japanese Classification of Colorectal Carcinoma [9].
D3 lymph node dissection requires complete dissection of the pericolic lymph nodes ≥ 10 cm from the tumor on either side and intermediate nodes as well as the removal of lymph nodes at the origin of the MCA [9]. The distribution of the retrieved node was assessed using the modified clearing method [11]. Recurrence was investigated through regular examinations, including office visits and assays of tumor markers every 3 months and computed tomography every 6 months for the first 5 years. The median follow-up period of recurrence-free patients was 61.0 months (range 22.3–112.9 months). The Kyushu University Hospital Human Research Ethics Committee approved this study (No. 26-239), and written informed consent on the laparoscopic resection was obtained from all patients before surgery.
Surgical anatomy of middle colic vessels
Here, we define MCAs as arteries that run cranioventrally in the TMC upon cranial lifting. The right branch of the MCA (right MCA) was bounded by the hepatic flexure and the right side of the transverse colon, while the left branch of the MCA (left MCA) supplied blood for the middle to the left side of the transverse colon. The branch of the superior mesenteric artery (SMA) running behind the body of the pancreas directed to the left portion of the TMC was defined as an accessory MCA (Ac MCA) [22, 23]. We defined a draining vein that parallels the left MCA as the left-middle colic vein (left MCV), and the right-middle colic vein (right MCV) was designated as the vein carrying blood along the right MCA. In the present study, the vessels running through the ascending mesocolon, which joined directly to the SMA and SMV, were classified as a right colic artery (RCA) and vein (RCV), respectively, because it was difficult to identify the precise direction of the branch of the MCA or ileocolic artery (ICA) during arterial division during surgery.
Laparoscopic bidirectional dissection of the TMC during transverse colectomy (TC) and right hemicolectomy (RHC)
The greater omentum was laparoscopically dissected from the transverse colon, and the lesser sac was entered. The dorsal part of the greater omentum, which merged with the ventral surface of the TMC [5], was incised, and the right gastroepiploic vein as well as the gastrocolic trunk (GCT) were visualized (Fig. 1A). Next, the fusion between the caudal border of the pancreas body and the root of the TMC was divided, and separation of the mesocolon proceeded toward the head of the pancreas, exposing the surfaces of the SMV and GCT. These processes allowed us to identify the roots of the right and left MCVs and to transect them at their origins (Fig. 1B). After continuing the separation of the mesocolon from the surface of the pancreas and duodenum to the right side, the hepatic flexure was mobilized. The left part of the greater omentum was dissected, the fusion of the dorsal part of the omentum and the TMC was separated from the caudal border of the pancreas tail, and the splenic flexure was mobilized, if required. The transverse colon was subsequently lifted caudally, and the dorsal surface of the TMC appeared.
Intraoperative view of a transverse colectomy. A Dissection the greater omentum and the right MCV. B After dissection of the caudal border of the TMC and exposing the SMV, the left MCV was divided. C After upending the TMC caudally, the gently sloping “U”-shaped incision was made. D The surfaces of the SMV and SMA were exposed, and the root of the MCA was identified. Arrowheads indicate the border of the TMC. TMC transverse mesocolon, RGEV right gastroepiploic vein, right MCV right middle colic vein, left MCV left middle colic vein, MCA middle colic artery, SMV superior mesenteric vein, SMA superior mesenteric artery
During TC, the gently sloping “U”-shaped incision on the peritoneum of the root of the TMC was made to connect the translucent area above the second portion of the duodenum to that above the duodenojejunal flexure; and the dorsal and ventral dissections of the TMC were then bilaterally joined (Fig. 1C). After clearing the lympho-adipose tissue above the SMV and SMA, the root of the MCA was identified and transected at its origin (Fig. 1D). Finally, the bottom of the TMC was completely dissected from the pancreas and the ventral surfaces of the SMV and SMA. Before minilaparotomy, the proximal and distal sides of the mesocolon were bilaterally incised 10 cm from the tumor.
During RHC, the peritoneal surface of the end of ileal mesentery was incised parallel to the ICA and the ileocolic vein (ICV), extending to the duodenojejunal flexure along the left side of the SMA. The ICA and ICV as well as the RCA and RCV were identified and transected at their origins after removing the lympho-adipose tissue on the surfaces of the SMV and SMA. The origin of the MCA was identified, and the right MCA was divided after complete removal of the lympho-adipose tissue around the origin of the MCA to its bifurcation. Medial-to-lateral dissection of the right colon and the division of the TMC 10 cm from the tumor were performed before minilaparotomy.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Specifically, EZR is a modified version of R commander designed to add statistical functions frequently used in biostatistics [24]. Patients’ clinical and demographic characteristics were analyzed using χ2 tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. Survival analysis was conducted using the Kaplan–Meier method and the log-rank test. All P values were two sided, and P values ≤ 0.05 were considered statistically significant.
Results
Clinicopathological findings and surgical outcomes
Of the 42 patients who underwent D3 resection for TCC, 20 (47.6%) underwent TC, including patients with partial resection of the descending colon, and 21 (52.4%) underwent RHC. One patient with a malignant lymph node surrounding the abdominal aorta underwent radical surgery involving removal of the para-aortic nodes. A patient with familial adenomatous polyposis with advanced transverse colon cancer and two small hepatic metastases in segment IV of the liver underwent proctocolectomy and partial resection of the liver. Three patients underwent simultaneous major operations, including pancreatoduodenectomy for intraductal papillary mucinous neoplasms of the pancreas, anterior resection for concomitant rectal cancer, and subtotal esophagectomy for esophageal cancer. The clinicopathological findings are summarized in Table 1.
Patients’ pathological stages were as follows: T2, n = 3 (7.1%); T3, n = 34 (81.0%); T4a, n = 4 (9.5%); T4b, n = 1 (2.4%). Nodal status was as follows: N0, n = 20 (47.6%); N1a, n = 4 (9.5%); N1b, n = 14 (33.3%); N2a, n = 2 (4.8%), N2b, n = 2 (4.8%). The median number of lymph nodes retrieved lymph from the TMC was 18 (range 8–101), including 7 (0–71) pericolic, 5 (1–18) intermediate, and 5 (0–18) in the main node areas. Lymph node metastases in these three areas were present in 19 (45.2%), 10 (23.8%), and 5 (11.9%) of the 42 patients, respectively (Table 2). There was no significant association between tumor depth and the extent of lymph node metastases, whereas the rate of the lymph node metastases in the main area increased according to the number of lymph node metastases (Table 2). The resection margins of all patients were histologically free of tumor cells, including two patients with Stage IV disease.
Tumors recurred during follow-up in 11 (26.2%) of the 42 patients, including metastases in the liver (n = 5), lungs (n = 3), peritoneum (n = 3), and lymph nodes (n = 2). Among five patients with metastatic disease in their main lymph nodes, one patient with Stage IIIC neuroendocrine carcinoma developed lymph node recurrence around the SMA and multiple liver metastases, and died 20 months after surgery. The other patient underwent simultaneous partial hepatectomy of the hepatic metastases, received a second partial hepatectomy for metachronous liver metastases, and was alive 53 months after initial surgery. While one Stage IIIB patient died of cerebral infarction 8 months after surgery, the remaining two were alive without recurrent disease 54 months and 66 months after surgery.
Comparison of the outcomes of laparoscopic and open D3 surgeries
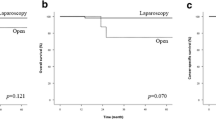
A comparison of the surgical outcomes between 36 patient who underwent laparoscopic colectomy with D3 dissection (Lap D3) and six patients who underwent open colectomy with D3 dissection (Open D3) is shown in Table 3. The median tumor size was larger in the Open D3 group compared with that of the Lap D3 group (P < 0.001). There were no significant differences in the pathological stage, type of surgery, and the duration of surgery. In contrast, blood loss was significantly lower in the Lap D3 group compared with that in the Open D3 group (median 26 mL vs 223 mL, P < 0.001). Two (5.6%) patients in the Lap D3 group required open conversion because of failure to detect the vessels and intraoperative hemorrhage. The number of the harvested lymph nodes did not significantly differ between the Lap and Open D3 groups (median 19.5 vs 17.5, P = 0.914). Postoperative mortality did not occur in either group, and there were 12 (28.6%) grade 2 or greater postoperative complications, including one anastomotic leakage, one intraabdominal abscess, three wound infections, five lymph leakages, one prolonged paralytic ileus, and one case of enteritis. The incidence of postoperative complications and the median postoperative hospital stay were not significantly different between the groups. The rates of tumor recurrence did not differ significantly between groups (25.0% vs 33.3%, P = 0.644), and 3-year overall survival rates were not significantly different between groups (87.7% vs 83.3%, P = 0.385).
Arterial anatomy
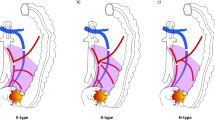
We propose four different patterns of MCA variation according to the number of MCAs and a supplementary AcMCA (Fig. 2). In pattern I, there is a solitary stem of the MCA that forks to the right and left as the right MCA and left MCA. In pattern II, there are two stems of the MCA from the SMA. In pattern III, one main stem of the MCA is present along with a supplementary AcMCA that originates from the SMA behind the pancreas. In pattern IV, there are two stems of the MCA and the AcMCA. All 42 patients had an MCA, and the frequency of each pattern is shown in Table 4. The frequencies of the patterns were as follows: Pattern I, n = 27 (64.3%); pattern II, n = 9 (21.4%); pattern III, n = 1 (2.4%); and pattern IV, n = 5 (11.9%) (Table 4). From a different perspective, the two stems of the MCA were present in 33.3% of resections, and the AcMCA was present in 14.3%.
Branching patterns of the middle colic artery (MCA). A (pattern I) Single MCA trunk arising from the superior mesenteric artery (SMA), forking right and left. B (pattern II) two stems of the MCA originating from the SMA. C (pattern III) Single main stem of the MCA and a supplementary accessory MCA (AcMCA). D (pattern IV) Two stems of the MCA and the AcMCA
Venous anatomy
Most (92.9%) of the right MCVs along with the right MCAs drained into the GCT, while the right MCV in three (7.1%) resections joined the left MCV and flowed into the SMV. The numbers of right MCVs in the resections were as follows: one in 26 (61.9%), two in 13 (31.0%), and three in three (7.1%). Further, the left MCV was a tributary mainly of the SMV in 38 (90.5%) patients, the splenic vein in two (4.8%), the GCT in one (2.4%), and the jejunal vein in one (2.4%). There were one and two left MCVs in 36 (85.7%) and 6 (14.3%) resections, respectively. Subsequently, the root of the transverse mesocolon extended from the confluence where the right MCV and the GCT meet, and the junction of the left MCV and SMV to the divergence of the MCA from the SMA and was broadly attached to the head of the pancreas and to the SMV/SMA (Fig. 3A, B).
Schematic of the transverse mesocolon (TMC) from the left side of the body and intraoperative view. A The TMC including the middle colic vessels is enclosed by the black line. B After division of the middle colic vessels and D3 lymph node dissection, the pancreas and the SMV and SMA were exposed. Arrowheads indicate the extent of the root of the TMC. RGEV right gastroepiploic vein, T-colon transverse colon, ASPDV anterior superior pancreatoduodenal vein, right MCV right middle colic vein, left MCV left middle colic vein, right MCA right branch of the middle colic artery, left MCA left branch of the middle colic artery, SMA superior mesenteric artery, SMV superior mesenteric vein
Discussion
We describe our evaluation of bidirectional resection of the TMC. This procedure involved exposing the GCT and the caudal border of the body of the pancreas as well as dividing the MCV during the initial phase of surgery, after separating the gastrocolic ligament following the embryonal plane. These steps enabled the surgeon to excise the entire TMC at the cranial-most boundary, namely, the confluence of the GCT and the right MCV to the surface of the SMV. The subsequent caudal-to-cranial dissection of the TMC and division of the MCA at the root was safely performed without jeopardizing the venous walls or the pancreas (Fig. 1D).
Identifying the origin of each MCV and lifting the entire base of the TMC out of the SMV and SMA may be difficult using a caudal-to-cranial or medial approach [25], because the TMC frequently adheres to the gastric wall. Moreover, it may be challenging to detect the MCV, because it is located behind the MCA. In these meticulously performed processes, a better viewing angle and augmented imaging provided by the laparoscope can be advantageous. Although the number of patients in our series was small, and the median size of the tumors was smaller in the Lap D3 group compared with that of the Open D3 group, blood loss caused by surgery was lower in the former, which supports our conclusions.
The mean (22.1) and median (19.5) numbers of lymph nodes retrieved using laparoscopic D3 was larger compared with those reported by previous retrospective studies (mean, 17.5; median, 12) [19, 26]. This may be explained by the inclusion in these studies of low vessel ligation, i.e., D2 lymph node dissection [26] or “en-bloc” vascular ligation, leaving the node in the D3 area [19]. During the Japanese D3 protocol, complete removal of the mesocolon according to precise surgical anatomy is essential, because D3 surgeries confer a significant survival benefit upon patients with curable colon cancer patients compared with those who undergo low vessel resection (D2) [27, 28]. Moreover, the surgeon must provide an adequate specimen composed of the segment of bowel and its accompanying intact mesentery to the level of the origin of the supply and draining vessels, which achieves complete evaluation of the lymph node basin [29].
The median number of retrieved main lymph nodes of the TMC in the D3 area was five (range 0–18), and five (11.9%) of the 42 patients had metastasized nodes in this area. These nodes were present around the confluence of the right MCV and GCT or over the SMV and SMA, which may have been misinterpreted as extraregional lymph nodes such as infrapyloric nodes, gastrocolic ligament nodes, or infrapancreatic nodes [30,31,32]. Although the five patients had relatively advanced disease (one with Stage IIIB, two with Stage IIIC, and two with Stage IV), they all underwent curative R0 surgery and three survived longer than 50 months after surgery. Thus, we recommend removal of these regional nodes to achieve a cure as well as for accurate lymph node staging and delivery of effective adjuvant chemotherapy [31,32,33]. Moreover, these complete mesenteric excisions improve 3-year survival, even for patients with early-stage (I–II) colon cancers compared with D2 resection [28], likely because incomplete dissection or disruption of the mesocolon package may lead to spilling tumor cells over the surgical fields.
The most significant concerns associated with complete dissection are perioperative adverse events and delay of postoperative recovery [34]. The conversion rate of the LAP D3 group was 5.6% compared with 5.4% reported by a Japanese multicenter prospective study that compared surgical outcomes between laparoscopic and open D3 dissection for advanced colon cancers, excluding TCC [8]. The median operating time in our series was comparable to that of this study [8] and those of retrospective studies of laparoscopic D3 for TCC [26, 35], although longer compared with those of other studies that did not report the precise level of vascular ligation [19, 36], indicating the technical difficulty of D3 dissection for advanced colon cancer. The median blood loss (26 mL) was less compared with those of other studies [19, 26, 36], and members of our LAP D3 group did not receive transfusions.
Although the incidences of postoperative morbidity (27.8% and 33.3% for LAP D3 and Open D3, respectively) were relatively higher compared with those of retrospective studies that included D2 dissection [19, 26, 36], none the patients suffered an organ injury or a pancreatic fistula requiring further surgery or longer hospitalization. All postoperative complications were resolved using noninvasive treatment. The median postoperative hospital stay (10 days) was almost identical than those of the retrospective Japanese D3 [8] and Korean studies [26], although longer than those of retrospective studies conducted in Western countries [18, 19]. These findings suggest that factors other than treatment influence time-to-discharge, such as medical and insurance expenses. Notably, these results suggest that minimally invasive LAP D3 can be performed as safely for advanced transverse colon cancers as for cancers in other locations.
Insufficient information is available about the variations of the vessels in the transverse colectomy [20, 37]. In contrast, our inspection and discrimination of surgical anatomy reported here enhances our understanding of drainage patterns of the transverse mesocolonic veins, which are important for performing D3 lymph node dissection, because the lymphatic flows run along with the arteries and parallel to the veins [30, 38]. The main draining veins were designated the right and left MCVs according to their associated arteries. This designation was helpful when either of the MCA branches was preserved during right or left hemicolectomy. Importantly, the right MCV is mainly a tributary of the GCT, not the SMV, which may explain why advanced TC sometimes metastasizes to the lymph nodes along the GCT. Further, multiple right and left MCVs are occasionally present.
Although each MCV mainly streams parallel to the MCA at the periphery, the location of their roots are not close to those of the corresponding MCAs. Because of this inconsistency, the base of the TMC was widely distributed on the pancreas head to the surfaces of the SMA and SMV (Fig. 3A, B). These results are largely consistent with those of a previous report using cadavers [20, 37]. Thus, simple ligation of the MCA without considering the accompanying MCV would leave the main lymph nodes unresected but jeopardizes the integrity of the MCVs. Possible injuries to these vessels followed by continuous bleeding would require an open conversion during laparoscopic surgery, which is associated with poor oncological and short-term outcomes [39]. The frequency of occurrence of two stems of the MCA was greater compared with those previously reported [37, 40, 41], which may be explained by our definition of the auxiliary artery heading to the hepatic flexure as the right or secondary right MCA. Further, the anatomy of patients’ colonic vessels during surgery that requires pulling the TMCs caudually or lifting them cranially during the different steps of the procedures may differ from that revealed by imaging systems before surgery, because the TMC is folded and overlaid on the pancreas [42].
In conclusion, laparoscopic D3 lymph node dissection using bidirectional dissection of the TMC is feasible, and knowledge of vascular anatomy may provide improved technical and oncological safety for the treatment of advanced TCC. Our present retrospective study included an insufficient number of patients from which to draw definitive conclusions. However, our findings provide compelling evidence that progress in improving surgical techniques and innovations in pre- and intraoperative imaging will likely decrease surgical and postoperative complications. Further prospective studies comparing short- and long-term outcomes of patients are required to validate the efficacy of the laparoscopic D3 lymph node dissection. We are convinced that systematized, higher quality surgery decreases the rate of local and peritoneal recurrences and improves patients’ outcomes.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 11:354–364; discussion 364 – 355
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Culligan K, Coffey JC, Kiran RP, Kalady M, Lavery IC, Remzi FH (2012) The mesocolon: a prospective observational study. Colorectal Dis 14:421–428; discussion 428–430
Mike M, Kano N (2014) Laparoscopic surgery for colon cancer: a review of the fascial composition of the abdominal cavity. Surg Today. 45:129–39
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM, Group COcLoORS (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. The Lancet Oncol 6:477–484
Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S, Japan Clinical Oncology Group Colorectal Cancer Study G (2014) Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg 260:23–30
Japanese Society for Cancer of the Colon and Rectum (2013) Japanese classification of colorectal carcinoma. The 8th edition. Kanehara & CO, LTD, Tokyo
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K, Japanese Society for Cancer of the C, Rectum (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239
Morikawa E, Yasutomi M, Shindou K, Matsuda T, Mori N, Hida J, Kubo R, Kitaoka M, Nakamura M, Fujimoto K et al (1994) Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon Rectum 37:219–223
Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K (1997) The extent of lymph node dissection for colon carcinoma: the potential impact on laparoscopic surgery. Cancer 80:188–192
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30:1763–1769
Akiyoshi T, Kuroyanagi H, Fujimoto Y, Konishi T, Ueno M, Oya M, Yamaguchi T (2010) Short-term outcomes of laparoscopic colectomy for transverse colon cancer. J Gastrointest Surg 14:818–823
Yamamoto M, Okuda J, Tanaka K, Kondo K, Tanigawa N, Uchiyama K (2012) Clinical outcomes of laparoscopic surgery for advanced transverse and descending colon cancer: a single-center experience. Surg Endosc 26:1566–1572
Mulder SA, Kranse R, Damhuis RA, Ouwendijk RJ, Kuipers EJ, van Leerdam ME (2012) The incidence and risk factors of metachronous colorectal cancer: an indication for follow-up. Dis Colon Rectum 55:522–531
Battersby NJ, Coupland A, Bouliotis G, Mirza N, Williams JG (2014) Metachronous colorectal cancer: a competing risks analysis with consideration for a stratified approach to surveillance colonoscopy. J Surg Oncol 109:445–450
Fernandez-Cebrian JM, Gil Yonte P, Jimenez-Toscano M, Vega L, Ochando F (2013) Laparoscopic colectomy for transverse colon carcinoma: a surgical challenge but oncologically feasible. Colorectal Dis 15:e79–e83
Mistrangelo M, Allaix ME, Cassoni P, Giraudo G, Arolfo S, Morino M (2014) Laparoscopic versus open resection for transverse colon cancer. Surg Endosc. 29:2196–2202
Stelzner S, Hohenberger W, Weber K, West NP, Witzigmann H, Wedel T (2015) Anatomy of the transverse colon revisited with respect to complete mesocolic excision and possible pathways of aberrant lymphatic tumor spread. Int J Colorectal Dis. 31:377–84
Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti D (2010) AJCC cancer staging manual. Springer, New York
Koizumi M, Horiguchi M (1990) Accessory arteries supplying the human transverse colon. Acta Anat (Basel) 137:246–251
Amonoo-Kuofi HS, el-Badawi MG, el-Naggar ME (1995) Anomalous origins of colic arteries. Clin Anat 8:288–293
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH (2012) Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc 26:3669–3675
Kim MK, Won DY, Lee JK, Kang WK, Kye BH, Cho HM, Kim HJ, Kim JG (2015) Laparoscopic surgery for transverse colon cancer: short- and long-term outcomes in comparison with conventional open surgery. J Laparoendosc Adv Surg Tech A 25:982–989
Kotake K, Mizuguchi T, Moritani K, Wada O, Ozawa H, Oki I, Sugihara K (2014) Impact of D3 lymph node dissection on survival for patients with T3 and T4 colon cancer. Int J Colorectal Dis 29:847–852
Storli KE, Sondenaa K, Furnes B, Nesvik I, Gudlaugsson E, Bukholm I, Eide GE (2013) Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I–II. Tech Coloproctol. 18:557–564
Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA (2007) Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 99:433–441
Toyota S, Ohta H, Anazawa S (1995) Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum 38:705–711
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Jepsen LV, Kristensen B, Neuenschwander AU, Gogenur I (2014) Lymph node metastases in the gastrocolic ligament in patients with colon cancer. Dis Colon Rectum 57:839–845
Perrakis A, Weber K, Merkel S, Matzel K, Agaimy A, Gebbert C, Hohenberger W (2014) Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int J Colorectal Dis 29:1223–1229
Ricciardi R, Baxter NN (2007) Association versus causation versus quality improvement: setting benchmarks for lymph node evaluation in colon cancer. J Natl Cancer Inst 99:414–415
Schlachta CM, Mamazza J, Poulin EC (2007) Are transverse colon cancers suitable for laparoscopic resection? Surg Endosc 21:396–399
Mori S, Kita Y, Baba K, Yanagi M, Tanabe K, Uchikado Y, Kurahara H, Arigami T, Uenosono Y, Mataki Y, Okumura H, Nakajo A, Maemura K, Natsugoe S (2017) Laparoscopic complete mesocolic excision via combined medial and cranial approaches for transverse colon cancer. Surg Today 47:643–649
Hirasaki Y, Fukunaga M, Sugano M, Nagakari K, Yoshikawa S, Ouchi M (2014) Short- and long-term results of laparoscopic surgery for transverse colon cancer. Surg Today 44:1266–1272
Yamaguchi S, Kuroyanagi H, Milsom JW, Sim R, Shimada H (2002) Venous anatomy of the right colon: precise structure of the major veins and gastrocolic trunk in 58 cadavers. Dis Colon Rectum 45:1337–1340
Spasojevic M, Stimec BV, Dyrbekk AP, Tepavcevic Z, Edwin B, Bakka A, Ignjatovic D (2013) Lymph node distribution in the d3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Dis Colon Rectum 56:1381–1387
Clancy C, O’Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, Myers E (2014) A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery: the official journal of the Association of Coloproctology of Great Britain and Ireland. Colorectal Dis. 17:482–490
Ignjatovic D, Sund S, Stimec B, Bergamaschi R (2007) Vascular relationships in right colectomy for cancer: clinical implications. Tech Coloproctol 11:247–250
Spasojevic M, Stimec BV, Fasel JF, Terraz S, Ignjatovic D (2011) 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surg Endosc 25:1883–1886
Spasojevic M, Stimec BV, Gronvold LB, Nesgaard JM, Edwin B, Ignjatovic D (2011) The anatomical and surgical consequences of right colectomy for cancer. Dis Colon Rectum 54:1503–1509
Acknowledgements
We thank Edanz Group (https://www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Ueki, Nagai, Manabe, Koba, Nagayoshi, and Nakamura have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Ueki, T., Nagai, S., Manabe, T. et al. Vascular anatomy of the transverse mesocolon and bidirectional laparoscopic D3 lymph node dissection for patients with advanced transverse colon cancer. Surg Endosc 33, 2257–2266 (2019). https://doi.org/10.1007/s00464-018-6516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6516-2