Abstract
Background
Robotic surgery promises to extend the capabilities of the minimally invasive surgeon. The aim of this study was to examine the feasibility of robotic surgery in the setting of laparoscopic gastric bypass.
Methods
The Zeus robotic surgical system was used in 50 laparoscopic gastric bypass procedures. The learning curve was staged to add complexity to the robotic tasks as experience grew. Robotic setup time, robotic operative time, total operative time, and operative outcomes were tracked prospectively.
Results
We observed a significant decrease in the robotic setup time. Our robotic learning curve demonstrated decreased operative time, even as more complex tasks were accomplished. Total operative time also decreased significantly over the series. There were no complications in our series that could be attributed to the robotic technique.
Conclusions
Robot-assisted laparoscopic Roux-en-Y gastric bypass is safe. The steadiness and extra degrees of freedom of surgical robotic systems may improve the accuracy of laparoscopic tasks. The learning curve for robot-assisted laparoscopic Roux-en-Y gastric bypass is significant but manageable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Morbid obesity has grown to epidemic proportions worldwide over the past decade [11, 16, 17]. Minimally invasive techniques have been applied to a variety of bariatric surgical procedures, including vertical banded gastroplasty, adjustable gastric banding, biliopancreatic diversion with duodenal switch, and Roux-en-Y gastric bypass (RYGB).
Several large series have demonstrated the safety and efficacy of laparoscopic RYGB [2, 4, 12, 15], as well as a shorter recovery time, decreased pain, and fewer complications compared to open gastric bypass [5, 7]. These hallmarks of minimally invasive surgery have made laparoscopic RYGB more widely accepted and more attractive to patients requiring obesity surgery.
From a surgeon’s perspective, however, laparoscopic RYGBP has introduced new technical challenges, demanding skill levels beyond routine laparoscopic surgical procedures (e.g., cholecystectomy and appendectomy) or even advanced laparoscopic procedures. Furthermore, the learning curve for mastering this technique and optimizing surgical outcomes can be quite steep [9, 13].
For decades, industry has used robots successfully for fine, delicate, repetitive tasks. More recently, these features of robotics have been introduced into the surgical suite. With the introduction of AESOP (Automated Endoscopic System for Optimal Positioning; Computer Motion, Santa Barbara, CA, USA), surgical robots were first placed into mainstream clinical use as laparoscope holders under direct command of the operating surgeon. Their use enabled a steady image to be maintained, with the added benefit that one less surgical assistant was needed at the operating table.
Further advances in robotic technology have made it possible for the surgeon’s hand movements to be translated from handles located at a console to robotic arms at the operative field, enabling remote performance of surgical tasks in a safe and precise manner. Although there have been many laboratory trials [1, 3, 8], clinical experience with surgical robots remains limited [6, 14]. The applicability of this technology to various surgical procedures has ranged from delicate and meticulous applications (e.g., minimally invasive cardiac surgery and nerve-sparing radical prostatectomy) to macrosuturing applications (e.g., intestinal anastomosis) and gross dissection (e.g., laparoscopic cholecystectomy), as demonstrated in laboratory settings. Due to their versatility and advanced technical capabilities, surgical robots promise to expand the laparoscopic capabilities of both the seasoned laparoscopic surgeon and the novice.
The FDA approval of the Zeus surgical robotic system (Computer Motion, Santa Barbara, CA, USA) for gastrointestinal surgery prompted us to challenge its clinical efficacy and reliability in the setting of one of the most complex laparoscopic procedures currently performed, the laparoscopic RYGB. We sought to demonstrate the feasibility of applying robotic surgical technology to gastric bypass surgery. We present our series of robot-assisted laparoscopic RYGB using the Zeus surgical robotic system.
Materials and methods
Patient selection
Between December 2002 and March 2003, Zeus-assisted laparoscopic RYGB was performed in 50 patients at the University of California-Davis Medical Center. During this period, a total of 56 laparoscopic RYGB were performed. Robotic RYGB was not performed on two patients due to the following patient characteristics: inadequate length of robotic instruments in a tall patient and transient hypotension in another patient, which would not have justified the extra time required to set up and use the robot. The remaining four patients were excluded due to technical factors with the robotic system that made it unavailable for use on a particular case. There were no preoperative patient-specific inclusion or exclusion criteria.
Technique
We use a six-incision technique for laparoscopic RYGB. The liver retractor is placed through a 5-mm subxyphoid incision, and the laparoscope is placed through a periumbilical trocar. The remaining port configuration enables the surgeon and the surgical assistant to each operate with two hands.
In developing our robotic technique, we adapted the setup of the robotic system so as to preserve the same trocar position used in conventional laparoscopic RYGB. This has conferred two important advantages to our technique: (a) equal and easy access to operative targets within the lower abdomen and the upper abdomen and (b) seamless exchange between robotic and conventional laparoscopic technique in the performance of any specific surgical task.
We perform a side-to-side GIA-stapled jejunojejunostomy with a retrocolic, retrogastric jejunal Roux limb. All mesenteric defects and the Petersen defect (posterior space between the transverse mesocolon and the small bowel mesentery) are carefully closed. The gastrojejunostomy is a two-layer anastomosis, the outer layer of which is a circumferential running sewn layer. The inner layer consists of a GIA-stapled side-to-side posterior row with a running sewn enterotomy closure. This anastomosis is tested intraoperatively by endoscopic insufflation under external saline submersion.
The robotic setup for Zeus-assisted laparoscopic RYGB is shown in Fig. 1. The surgeon is seated remotely from the operative field at the control console (Fig. 1a). The robotic instruments are inserted through the right subcostal and the right upper quadrant trocars (Fig. 1b). The green instrument positioner (IP) of the Zeus system is placed on the left side of the operating table and crosses the patient to control the instrument in the surgeon’s “left hand.” The yellow IP is placed on the right side of the operating table and controls the instrument in the surgeon’s “right hand.” Finally, the laparoscope is directed via the periumbilical trocar by the robotic scope holder, AESOP, under voice control by the surgeon. We have found that this configuration greatly reduces external conflict of the robotic arms and enhances the comfort and effectiveness of the surgical assistant (Fig. 1c).
Implementation of robotics
The gastrojejunal anastomosis in gastric bypass surgery requires precise surgical technique. We staged the development of the robotic procedure to ensure patient safety as the learning curve progressed, with the ultimate goal of constructing a precise robotic gastrojejunostomy (Fig. 2). The first four cases were dedicated to defining the logistics of robotic positioning and to gaining experience with the robot. As such, we chose a relatively simple surgical task (suturing the jejunojejunal mesenteric defect) to determine the early feasibility of this technology. Over the next eight cases, robotic positioning was refined to enable performance of surgical tasks in both the lower abdomen and the upper abdomen using a single robotic arm configuration. The remainder of the cases were dedicated to perfecting the technique of robotic gastrojejunostomy (GJ).
Data collection
All data were collected prospectively into a well-defined scheme. We tracked robotic setup time, robotic operative time, and total operative time. The occurrence of any surgical complications was carefully documented. Data were analyzed using the Student t-test for pairwise comparisons and analysis of variance (ANOVA) to test differences among multiple groups. Statistical significance was set at α = 0.05 for all comparisons.
Results
Zeus-assisted laparoscopic RYGB was performed on 50 patients during the study period (Table 1). The vast majority of patients in this series were female, consistent with the gender distribution in this patient population. Patients ranged in body mass index (BMI) from 40 to 64 kg/m2. Although most patients had a BMI < 50, a significant number of patients were super-obese (BMI > 50). Many patients had undergone previous abdominal operations.
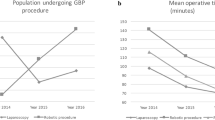
To ascertain the utility of the robotic system in routine practice, we tracked robotic setup time for all cases. At the beginning of each case, the robotic arms were draped and positioned onto the railings of the operating table. Once we began the robotic portion of the operation, the initial robotic setup time was measured as the time required to realign the liver retractor, configure the robotic arms into the operating position, attach motor packs and instrument drivers, and bring the robotic surgical instruments and the laparoscope into the surgical field intracorporeally. In cases where the Zeus system was used more than once, a secondary setup time was also recorded. As expected, robotic setup time for the first case (initial setup only) was long (30.3 min). The time required to set up the Zeus system decreased dramatically over this series of cases, so that the total setup time (initial + secondary) for the final case in the series was 3.88 min and the fastest total setup time (initial + secondary) was 2.13 min. The mean total robotic setup time in cases 1-4 (closure of mesenteric defect, initial setup only) was 18.8 vs 2.9 min in cases 47-50 (complete GJ, initial + secondary setup) (p = 0.01) (Fig. 3).
To further assess the clinical efficacy and safety of surgical robots in laparoscopic RYGB, the time required to complete robotic surgical tasks was carefully followed. Robotic operative times are reported as the sum of duration of all robotic tasks for each case (e.g., mesenteric closure required a single robotic task, whereas the creation of a GJ required three robotic tasks, one for each layer). We observed remarkable improvement in robotic operative technique such that completion of a complex robotic task (complete GJ) at the end of the study (cases 47-50, mean = 51.8 min) required less time than a simpler task (mesenteric closure) at the beginning of the series (cases 1-4, mean = 57.7 min) (Fig. 3). Although this result did not reach statistical significance, it has clear clinical significance in the context of the increased complexity of the surgical task performed.
Moreover, this study demonstrated the learning curve for robot-assisted gastric bypass (Fig. 4). Improvement in robotic operative time was observed in rather short intervals (five cases). Naturally, these times increased whenever more complex surgical tasks were added but such plateaus in total robotic operative time were readily overcome as robotic operative experience accumulated (figure 4). Although these decreased robotic operative times did not reach statistical significance by ANOVA, there was a strong trend toward statistical significance between group 4 and group 10. The time required to construct a robotic GJ was significantly longer than the average time required to perform a standard laparoscopic GJ (20 min), even late in the robotic experience (group 10). Total operative time also decreased significantly as our experience grew from the first 10 cases (316 ± 55 min) to the final 10 cases (232 ± 51 min) (p = 0.001).
Most important, there were no complications attributable to the robotic technique in this series. A single intraoperative leak occurred at the GJ and was discovered by the air-leak test. In this case, the anastomosis had been created using standard laparoscopic technique (without the Zeus robot). We observed no anastomotic stricture at the robotic GJ, a rate identical to that of our standard laparoscopic technique.
Discussion
The increasing demand for minimally invasive surgical procedures has posed new challenges for surgeons seeking expertise in advanced laparoscopy. The introduction of robotic surgery may broaden the spectrum of laparoscopically -feasible procedures and extend the laparoscopic capability of today’s surgeon. Our findings indicate that the robot promises to overcome some of the current limitations of standard laparoscopy by offering the following features: (a) more degrees of freedom, (b) increased precision, (c) instrument stability, (d) image stability, (e) surgeon comfort, and (f) potential for telesurgery.
Perhaps one of the most common challenges for a novice laparoscopic surgeon is tying knots with the standard fixed, straight laparoscopic instruments. The articulation at the tip of the robotic instrument significantly facilitates the maneuvers required for knot-tying. This feature also enables manipulation of the instrument into angles that would otherwise be difficult to obtain using standard laparoscopic instrumentation, thus enabling the precise movements that are critical for demanding tasks such as the suturing of anastomoses and meticulous dissection. In addition, instrument stability obtained by down-scaling the surgeon’s hand movements further enables delicate and precise suturing and tissue-handling.
The ability to maintain a steady image is key in laparoscopic surgery, because minor movements of the camera can result in major shifts in the image. With AESOP, the camera is held steady. Although we did not use the three-dimensional imaging system for our cases, that feature may facilitate the surgeon’s maneuverability.
During a robotic case, the surgeon is seated comfortably at the control console. This may enhance the surgeon’s performance. The further addition of an extra robotic arm, to be used as a surgical assistant, may make it possible for a single surgeon to perform an entire case. However, the potential for “solo surgery” is limited by the procedure being performed. In our gastric bypass series, two surgeons were present at all times during the case, one at the control console and the other at the surgical field.
Remote telesurgery is a logical extension of robotic surgery. Recent reports from Canada have shown that remote surgery can be performed safely [10]. This application, however, will likely require some time to become clinically practical.
Some of the disadvantages that were noted early in our learning curve, were increased operative time, “separation anxiety” (because the surgeon is remote from the patient), and the absence of tactile sensation. We found that, with experience, the time factor and separation anxiety essentially disappeared. As for the lack of haptic feedback, with increasing experience in robotic surgery, visual cues become virtual tactile sensation.
Our early experience with robotic surgery has demonstrated that this technology can be used to perform the GJ in laparoscopic RYGB, with minimal additional risk to the patient in terms of technical problems or anastomotic complications. As the technology matures, robotic surgery may eventually make it possible for even the novice in minimally invasive surgery to perform advanced laparoscopic procedures. The learning curves may become less steep, thus enabling more surgeons to provide complex laparoscopic surgical services to patients. With increasing clinical experience, the applications for robotic surgery may be further extended.
References
L Chang RM Satava CA Pellegrini MN Sinanan (2003) ArticleTitleRobotic surgery: identifying the learning curve through objective measurement of skill Surg Endosc 17 1744–1748 Occurrence Handle10.1007/s00464-003-8813-6 Occurrence Handle1:STN:280:DC%2BD2c%2Fitl2isw%3D%3D Occurrence Handle12958686
EJ DeMaria HJ Sugerman JM Kellum JG Meador LG Wolfe (2002) ArticleTitleResults of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity Ann Surg 235 640–647 Occurrence Handle10.1097/00000658-200205000-00005 Occurrence Handle11981209
JD Hernandez SD Bann Y Munz K Moorthy V Datta S Martin A Dosis et al. (2004) ArticleTitleQualitative and quantitative analysis of the learning curve of a simulated surgical task on the da Vinci system Surg Endos 2 .
KD Higa T Ho KB Boone (2001) ArticleTitleLaparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up J Laparoendosc Adv Surg Tech 11 377–382 Occurrence Handle10.1089/10926420152761905 Occurrence Handle1:STN:280:DC%2BD38%2Fos1Gltw%3D%3D
JA Lujan MD Frutos Q Hernandez R Liron JR Cuenca G Valero P Parrilla (2004) ArticleTitleLaparoscopic versus open gastric bypass in the treatment of morbid obesity: a randomized prospective study Ann Surg 239 433–437 Occurrence Handle15024302
J Marescaux MK Smith D Folscher F Jamali B Malassagne J Leroy (2001) ArticleTitleTelerobotic laparoscopic cholecystectomy: initial clinical experience with 25 patients Ann Surg 234 1–7 Occurrence Handle10.1097/00000658-200107000-00001 Occurrence Handle1:STN:280:DC%2BD3MzlvFCisg%3D%3D Occurrence Handle11420476
NT Nguyen C Goldman CJ Rosenquist A Arango CJ Cole SJ Lee BM Wolfe (2001) ArticleTitleLaparoscopic versus open gastric, bypass: a randomized study of outcomes, quality of life, and costs Ann Surg 234 279–289 Occurrence Handle10.1097/00000658-200109000-00002 Occurrence Handle1:STN:280:DC%2BD3MvotVymtA%3D%3D Occurrence Handle11524581
D Nio R Balm S Maartense M Guijt WA Bemelman (2004) ArticleTitleThe efficacy of robot-assisted versus conventional laparoscopic vascular anastomoses in an experimental model Eur J Vasc Endovasc Surg 27 283–286 Occurrence Handle10.1016/j.ejvs.2003.12.013 Occurrence Handle1:STN:280:DC%2BD2c%2FlvVOjsg%3D%3D Occurrence Handle14760597
D Oliak GH Ballantyne P Weber A Wasielewski RJ Davies HJ Schmidt (2003) ArticleTitleLaparoscopic Roux-en-Y gastric bypass: defining the learning curve Surg Endosc 17 405–408 Occurrence Handle10.1007/s00464-002-8820-z Occurrence Handle1:STN:280:DC%2BD3s7gs1ejuw%3D%3D Occurrence Handle12399853
A Pirisi (2003) ArticleTitleTelerobotics brings surgical skills to remote communities Lancet 361 1794–1795 Occurrence Handle10.1016/S0140-6736(03)13449-6 Occurrence Handle12781543
BJ Rolls (2003) ArticleTitleThe supersizing of America: portion size and the obesity epidemic Nutr Today 38 42–53 Occurrence Handle10.1097/00017285-200303000-00004 Occurrence Handle12698053
PR Schauer S Ikramuddin W Gourash R Ramanathan J Luketich (2000) ArticleTitleOutcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity Ann Surg 232 515–529 Occurrence Handle10.1097/00000658-200010000-00007 Occurrence Handle1:STN:280:DC%2BD3M%2Fhs1eitA%3D%3D Occurrence Handle10998650
PR Schauer S Ikramuddin G Hamad W Gourash (2003) ArticleTitleThe learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases Surg Endosc 17 212–215 Occurrence Handle10.1007/s00464-002-8857-z Occurrence Handle1:STN:280:DC%2BD3s%2Fms12qsA%3D%3D Occurrence Handle12457218
MA Talamini S Chapman S Horgan WS Melvin (2003) ArticleTitleA prospective analysis of 211 robotic-assisted surgical procedures Surg Endosc 17 1521–1524 Occurrence Handle10.1007/s00464-002-8853-3 Occurrence Handle12915974
AC Wittgrove GW Clark (1999) ArticleTitleLaparoscopic gastric bypass: a five-year prospective study of 500 patients followed from 3 to 60 months Obes Surg 9 123–143 Occurrence Handle10.1381/096089299765553340 Occurrence Handle10391724
HR Wyatt (2003) ArticleTitleThe prevalence of obesity Prim Care 30 267–279 Occurrence Handle14567147
T Zimmermann-Belsing U Feldt-Rasmussen (2004) ArticleTitleObesity: the new worldwide epidemic threat to general health and our complete lack of effective treatment Endocrinology 145 1501–1502 Occurrence Handle10.1210/en.2004-0078 Occurrence Handle1:CAS:528:DC%2BD2cXis1Sjtbs%3D Occurrence Handle15026374
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, M.R., BhaskerRao, B. & Wolfe, B.M. Robot-assisted laparoscopic Roux-en-Y gastric bypass. Surg Endosc 19, 468–472 (2005). https://doi.org/10.1007/s00464-004-8705-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-8705-4