Abstract
Background: The incorporation of new devices into surgical practice often requires that surgeons acquire and master new skills. We studied the learning curve for intracorporeal knot tying in robotic surgery. Methods: We developed an objective scoring system to evaluate knot tying and tested eight attending surgeons during 3 weeks of training on a surgical robot. Each performed intracorporeal knot tying tasks both before and after robotic skills training. These performances were compared to their laparoscopic knots and analyzed to determine and define skill improvement. Results: Baseline laparoscopic knot completion took 140 sec (range, 47–432), with a mean composite score of 77 (100 possible), whereas robotic knot tying took 390 sec, with a mean composite score of 40. After initial robotic training, times decreased by 65% to 139 sec and scores increased to 71. With more training, completion times and composite scores were improved and errors were reduced. Conclusion: Like any new technology, surgical robotics requires dedicated training to achieve mastery. Initially, even experienced laparoscopists may register an inferior performance. However, after adequate training, surgeons can exceed their laparoscopic performance, completing intracorporeal knots better and faster using robotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Modern laparoscopic surgery was made possible by the advent of the miniature computer chip television camera and the development of high-intensity light sources, which improved the visualization of the surgical field through enhanced illumination and better resolution. Once the advantages of minimally invasive surgery became apparent, laparoscopy became the preferred approach for many operations, including cholecystectomy and antireflux surgery [6]. However, for several reasons, the use of standard endoscopic technology has not been widely adopted in all areas of surgical practice. According to Ballantyne [1], the pitfalls of laparoscopic surgery include an unstable camera platform, the loss of degrees of freedom, two-dimensional imaging, and poor ergonomics for the surgeon. To overcome these limitations, computer-controlled robotic actuators are now being used as aids in surgical procedures, enabling greater accuracy, camera stability, and improved visualization, as well as better dexterity than can be achieved with conventional endoscopic surgery. Operations that were once not amenable to the standard hand-controlled endoscopic technique are currently being performed with computer-assisted robotic surgery.
By interposing a computer between the surgeon and the instrument, the surgeon’s movement can be translated electronically into the appropriate motion of the instrument. Motion scaling can amplify the surgeon’s movements or, conversely, minimize them as needed for very fine, detailed work. Although human hands tend to falter over time due to fatigue and tremor, computers can correct for these slight oscillations to filter tremor from the surgeon’s movements resulting in a steady robotic maneuver. Computers also remain steady for optimal camera positioning and control. Thanks to high resolution and digital enhancement, visualization is improved. Moreover, computers can help the surgeon to regain additional degrees of freedom through joint articulations that are lost with traditional endoscopic instruments. This provides increased dexterity while operating within a limited space. The potential for robotic surgery to retain all the advantages of minimally invasive surgery while mitigating the shortcomings of laparoscopy is evident.
As with any new device or technology, an initial learning curve must be overcome to achieve competence and mastery. Although this learning curve has been repeatedly described in laparoscopy [7, 10], it has not been well-defined for robotic surgery. Because laparoscopic intracorporeal knot tying is a complex, discrete task that requires training, practice, and skill [12, 14], we used this task to define an objective scoring system that would measure skill and assess the learning curve for robotic technique.
Materials and methods
Over a 3-week period, eight attending surgeons with varying laparoscopic expertise and no experience with clinical robotic surgery underwent ≥5 h of individualized training in surgical robotics using the ZEUS Surgical Robotic System (Computer Motion, Goleta, CA, USA). Each training session was supervised by a clinical instructor and lasted 2–3 h, with no more than one session per day. All surgeons had at least two sessions within the 3-week period. During the initial training session, each surgeon was taught how to use the system and given a didactic lesson on intracorporeal knot tying with instruction on the appropriate steps and potential errors. As part of this study, all surgeons tied an intracorporeal knot using laparoscopic techniques (without the robot) at the first session. This performance was used as the benchmark against which the robotic knots were compared. After the didactic session, but before any hands-on robotic skills training, an intracorporeal knot was tied using robotic techniques. After each training session, the robotic knot-tying task was repeated. All knot-tying tasks were recorded by digital video for analysis.
Laparoscopic knot-tying
The laparoscopic intracorporeal knots were tied in a training box with three preset ports sites. A standard 10-mm 30° two-dimensional telescope was used in the middle port site for visualization and controlled manually by an assistant. The same assistant was used for all laparoscopic knots. Each surgeon used 5-mm right- and left-curved needle drivers in each hand to approximate two sides of a rubber tube using a 2-0 silk suture on a V-20 needle (US Surgical Corporation, Norwalk, CT, USA) precut to 4 in in length.
The assistant introduced the needle and suture into the training box. The task began when the surgeon loaded the needle onto the needle driver. The needle had to enter on one side of the cut Latex tube and exit out the other side. The first knot was a surgeon’s knot, followed by two additional square knots, each in the opposite direction to the last.
Robotic knot-tying
The robotic intracorporeal knots were tied using the ZEUS Surgical Robotic System in the same training box used for the laparoscopic knots. A 10-mm 30° three-dimensional (3-D) endoscope (Vista Medical Technologies, Carlsbad, CA, USA) was used in the middle port site for visualization. This unique 3-D endoscope attaches to a twin (stereo) endoscopic camera that acquires left- and right-eye images for viewing stereoscopically. The 3-D image was displayed at the surgeon’s center monitor at the console and could be visualized with special glasses. The scope was voice-controlled by the surgeon using the AESOP system (Computer Motion). The dominant hand controlled a 5-mm Micro-Joint heavy needle driver, while the nondominant hand controlled a 5-mm Micro-Joint fenestrated grasper. Both robotic arms with the aforementioned instruments were placed in the training box. The surgeon was seated at the console with hand controls for both robotic arms and instruments, 3-D visualization, and voice control of the endoscope positioner.
The assistant introduced a 2-0 silk suture on a V-20 needle precut to 4 ins into the trainer box. Motion scaling was not used in this task. Using robotics, the surgeon performed the same intracorporeal knot-tying task as described for the laparoscopic technique. Robotic knot tying was performed and recorded prior to training and then at varying intervals throughout training.
Objective scoring system
An objective scoring system was developed by task deconstruction and analysis based upon a set of predetermined task goals [9]. The essential steps required to complete an intracorporeal knot successfully were identified. Each step was assigned relative value based on the complexity of the step. Steps were counted as either completed or not completed. The total possible raw score for the successful completion of every step was 100 points. Next, after reviewing 30 archived recordings of intracorporeal knot tying, six common, discrete errors that were made during the completion of this task were identified. Each was assigned a relative value based on the severity of the error. If the same error occurred more than once, the frequency of the error was multiplied by the relative value of the error. The final composite score was calculated by subtracting the error score from the raw score (Fig. 1). This score could be a positive or negative value. The maximum possible points for a successfully completed knot without any errors was 100. Each laparoscopic and robotic knot was evaluated using this objective scoring system.
Interrater reliability assessment
Task videos were analyzed by two different surgeon-observers using our objective scoring system. Results from each observer were compared to determine interobserver agreement of raw, error, and composite scores.
Data analysis
All digital videos were reviewed and scored by a single observer, who was blinded to the surgeon performing the task. The composite score and the knot completion time were used to objectively demonstrate surgical skill. Statistical analysis was performed using the Student t-est with SPSS 11.0 for Windows (SPSS, Chicago, IL, USA).
Results
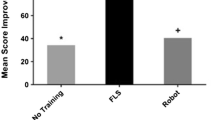
Prior to the initiation of robotic skills training, the completion of an intracorporeal knot using standard laparoscopic techniques took 140 sec (range, 47–432), which is significantly shorter than the average time of 390 sec (range, 201–603) for robotic knot tying without training (p < 0.05). Similarly, the mean composite score of the initial robotic knot was 40 (100 total points possible)—worse than the average laparoscopic composite score of 77. However, after only 4–6 h of robotic training, the average time to knot completion using robotics decreased by 65% to 139 sec (p < 0.05) and composite scores increased to 71. Times and scores at this point in training are similar to those recorded for the laparoscopic knot-tying task (NS). With even more training, in the range of 8–10 h, mean scores further increased to 94, while completion time dropped to 105 sec (p < 0.05), surpassing the baseline laparoscopic knots. This improvement continued after 14 h of training, when the mean composite score was 99 and time to completion was 82 sec (Figs. 2 and 3).
For each surgeon, the raw scores were 100 for nearly all knots. Error scores for robotic knots decreased with training (Fig. 4) and were highly correlated with completion time, with a Pearson’s coefficient of 0.93. The longer it took to complete the intracorporeal knot, the higher the error score (Fig. 5). Composite scores improved for each surgeon, except for one subject whose error score increased despite shorter completion times. All laparoscopic knots were performed faster than each surgeon’s respective pretraining robotic knot, except for one surgeon who had no experience in laparoscopic knot tying. In addition, the composite scores tended to be higher or equivalent for the laparoscopic technique. As the surgeons proceeded with the robotic skills training, the robotic knot completion time improved for all surgeons, eventually surpassing the laparoscopic knot completion time even for the two surgeons who had the least experience in intracorporeal knot tying.
After a review of several videos by two separate observers, the raw, error, and composite scores were compared. Interrater reliability was found to be high, with a p value >0.80.
Discussion
Laparoscopic intracoporeal knot tying is a complex, discrete task that has been validated in previous studies as an accurate test of skill [2]. It requires video–eye–hand coordination and bimanual dexterity. Although speed has traditionally been used as an objective measure of skill, we recognize that many other factors contribute to good technical ability. Therefore, we designed an objective scoring system for intracorporeal knot tying to assess skill. This score takes into account the completion of correct steps in knot tying as well as errors. Similar objective scoring systems have been described [3]. Completion times, as well as knot-tying scores, were used in this study to identify the learning curve for robotic surgery.
The analysis of the learning curves for robotic knot tying showed that the initial performance using the robotic system was inferior even for experienced laparoscopists. But after only 4–6 h of robotic skills training, robotic surgical performance in intracorporeal knot tying was comparable to that of standard laparoscopy. The greatest improvement was seen in the two surgeons who had the least experience in laparoscopic knot tying. Their completion times, composite scores, and error scores all quickly surpassed their laparoscopic performance after just minimal training. However, for surgeons who did have experience with intracorporeal knot tying, the robotic completion times matched but never superceded those for the laparoscopic knots.
One possible explanation for this result is that the lack of tactile feedback in the robotic systems results in dependence on visual cues to assess knot tension and tissue resistance. For subjects with no experience in reading these visual cues, errors such as suture breaking are more frequent, as are missed attempts to penetrate tissue with the needle. Operative times are therefore slower and composite scores are lower. As surgeons gain more experience and haptic technology improves, these limitations will be eliminated in succeeding generations of robotic systems.
Another potential reason why the robotic knot-tying times fell short of the laparoscopic times for experienced surgeons may be the choice of task. We opted to use 2-0 silk with a moderately sized needle for the intracorporeal knot. This task and suture is ideal for laparoscopy and does not require motion-scaling, which would likely not be of benefit in this task. In contrast, the reported advantages of robotic assistance are more appropriate for microsurgery. These include improved visualization, surgical stability, and dexterity, all of which are essential for fine surgical technique. In fact, Garcia-Ruiz et al. found that operative times for robotic suturing and tying decreased as suture size decreased from 2–0 to 7–0 suture while operative times actually increased in the same setting using laparoscopy [4].
In studies of surgically naïve subjects, the improvement in the learning curve has been shown to be more significant when robotics is used than with laparoscopy, suggesting that basic skills can be learned more quickly using robotics [13, 15]. Moreover, when Nio et al. studied the efficiency of manual laparoscopy vs robotic surgery in standardized tasks, including bead dropping, rope passing, needle capping, suturing, and cholecystectomy, they found that tasks using robotic assistance required fewer actions to complete [8]. This finding may have significant import for the future of surgical training, since there is growing public concern about the need for hands-on operative experience. As technology advances, these robotic systems will be used to complement box trainers and virtual reality simulators in the training of surgeons.
Interestingly, and perhaps most importantly, our study demonstrates that as surgeons progress through robotic skills training, not only do composite scores improve, but errors in intracorporeal knot tying are also decreased. In a report from the Institute of Medicine of the United States entitled, “To Err Is Human,” it was suggested that improved training and objective assessment would result in the reduction of errors, which would have a significant impact on patient safety [5]. Seymour et al. have also shown that technical training can reduce performance time and minimize errors, which subsequently translates into improved performance in the operating room [11].
In our study, errors in knot tying were identified, with the aim of developing an objective scoring system to assess surgical skill. With robotic training, our surgeons improved their skills by reducing both performance time and errors. This reduction in errors likely derives from the improved visualization and dexterity afforded by the robotic system. And ultimately, fewer errors means greater safety for our patients.
References
GH Ballantyne (2002) ArticleTitleThe pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 12 1–5 Occurrence Handle12008756
AM Derossis GM Fried M Abrahamowicz HH Sigman JS Barkun JL Meakins (1998) ArticleTitleDevelopment of a model for training and evaluation of laparoscopic skills. Am J Surg 175 482–487 Occurrence Handle10.1016/S0002-9610(98)00080-4 Occurrence Handle1:STN:280:DyaK1czgslOjuw%3D%3D Occurrence Handle9645777
TR Eubanks RH Clements D Pohl N Williams DC Schaad S Horgan C Pellegrini (1999) ArticleTitleAn objective scoring system for laparoscopic cholecystectomy. J Am Coll Surg 189 566–574 Occurrence Handle10.1016/S1072-7515(99)00218-5 Occurrence Handle1:STN:280:DC%2BD3c%2FlsFGisg%3D%3D Occurrence Handle10589593
A Garcia-Ruiz M Gagner JH Miller CP Steiner JF Hahn (1998) ArticleTitleManual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Arch Surg 133 957–961 Occurrence Handle10.1001/archsurg.133.9.957 Occurrence Handle1:STN:280:DyaK1cvitVaktA%3D%3D Occurrence Handle9749847
LT Kohn JM Corrigan M Donaldson (1999) To err is human: building a safer health system. Institute of Medicine Washington (DC)
MJ Mack (2001) ArticleTitleMinimally invasive and robotic surgery. JAMA 285 568–572 Occurrence Handle10.1001/jama.285.5.568 Occurrence Handle1:STN:280:DC%2BD3M7kvFKisw%3D%3D Occurrence Handle11176860
MJ Moore CL Bennett (1995) ArticleTitleThe learning curve for laparoscopic cholecystectomy. The Southern Surgeons Club. Am J Surg 170 55–59 Occurrence Handle10.1016/S0002-9610(99)80252-9 Occurrence Handle1:STN:280:ByqA3MzotVc%3D Occurrence Handle7793496
D Nio WA Bemelman KT Boer MS Dunker DJ Gouma TM Gulik (2002) ArticleTitleEfficiency of manual versus robotical (Zeus) assist laparoscopic surgery in the performance of standardized tasks. Surg Endosc 16 412–415 Occurrence Handle10.1007/s00464-001-9012-y Occurrence Handle1:STN:280:DC%2BD387psFSitg%3D%3D Occurrence Handle11928018
JC Rosser LE Rosser RS Savalgi (1997) ArticleTitleSkill acquisition and assessment for laparoscopic surgery. Arch Surg 132 200–204 Occurrence Handle1:STN:280:ByiC1MvkvV0%3D Occurrence Handle9041927
CM Schlachta J Mamazza PA Seshadri M Cadeddu R Gregoire EC Poulin (2001) ArticleTitleDefining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum 44 217–222 Occurrence Handle1:STN:280:DC%2BD3M7ot1Sksg%3D%3D Occurrence Handle11227938
NE Seymour AG Gallagher SA Roman MK O’Brien VK Bansal DK Andersen RM Satava (2002) ArticleTitleVirtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg 236 458–463 Occurrence Handle10.1097/00000658-200210000-00008 Occurrence Handle12368674
NJ Soper JG Hunter (1992) ArticleTitleSuturing and knot tying in laparoscopy. Surg Clin North Am 72 1139–1152
Strayer MP, Krause KR, Dalstrom DJ, Melvin WS (2001) Computer enhanced “robotic” telesurgery versus standard laparoscopy: performance on a skills test [Abstract]. Annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons (SAGES), St. Louis, MO, USA, April 2001
Z Szabo J Hunter G Berci J Sackier A Cuschieri (1994) ArticleTitleAnalysis of surgical movements during suturing in laparoscopy. Endosc Surg Allied Technol 2 55–61 Occurrence Handle1:STN:280:ByuA28zktl0%3D Occurrence Handle8081917
P Yohannes P Rotariu P Pinto AD Smith BR Lee (2002) ArticleTitleComparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology 60 39–45 Occurrence Handle10.1016/S0090-4295(02)01717-X
Acknowledgments
This study was supported in part by the US Surgical Corporation, a division of Tyco Healthcare, and by Computer Motion, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, L., Satava, R., Pellegrini, C. et al. Robotic surgery: identifying the learning curve through objective measurement of skill. Surg Endosc 17, 1744–1748 (2003). https://doi.org/10.1007/s00464-003-8813-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-003-8813-6