Abstract
Pharyngeal area can increase as a function of normal healthy aging and muscle atrophy. These increases in pharyngeal area can negatively affect swallowing function in healthy older adults (HOA). However, the presence of pharyngeal area changes and their effects on swallowing function in Parkinson’s disease (PD) remain unknown. Therefore, we compared the pharyngeal area of people with PD to HOA to determine if pharyngeal area changes were present in PD above and beyond what is seen in HOA. Within PD, we also evaluated if and how an increase in pharyngeal area affects swallowing kinematics, swallowing safety, and swallowing efficiency. A secondary analysis of videofluoroscopic swallow studies was completed comparing 41 HOA and 40 people with PD. Measures of pharyngeal area, swallowing kinematics, swallowing safety (penetration/aspiration), and swallowing efficiency (residue) were analyzed. An analysis of covariance (ANCOVA) was used to determine if pharyngeal area was significantly different between the HOA and PD groups while controlling for age, sex, and height. Regression analyses were used to examine if and how pharyngeal area influenced swallowing kinematics, swallowing safety, and swallowing efficiency in PD. Pharyngeal areas were significantly larger for people with PD when compared to HOA (p = .008). An increase in pharyngeal area was associated with less pharyngeal constriction (p = .022), shorter duration of airway closure (p = .017), worse swallowing safety (p < .0005), and worse swallowing efficiency (p = .037). This study revealed that pharyngeal areas are larger in people with PD when compared to HOA, and that this increase in pharyngeal area is associated with maladaptive changes to swallowing kinematics, residue, and penetration/aspiration. These findings support the notion that pharyngeal muscle atrophy may be exacerbated in PD above and beyond what is seen in normal, healthy aging group. Results from this study highlight the need to consider pharyngeal muscle atrophy as a source for swallowing dysfunction in PD, and as a potential treatment target for swallowing rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a highly prevalent neurodegenerative movement disorder that has been reported to result in dysphagia in approximately 82% of individuals at some point during the disease process [1]. Dysphagia increases total healthcare costs for affected hospitalized patients by an estimated 40% (~ $13,000 USD), increases hospital stays by approximately 3–5 days, and has a nationwide estimated economic impact of $547 million USD [2]. In addition, the presence of dysphagia increases the risk of developing serious medical conditions such as malnutrition, dehydration, and aspiration pneumonia [3]—a leading cause of death in PD [4].
The pathophysiology underlying dysphagia in PD is thought to be multifactorial in nature and caused by deficits to sensorimotor processing [5,6,7], motor control [8,9,10,11,12,13], and peripheral muscle weakness [14,15,16]. However, the relative contribution of each of these to disordered swallowing outcomes in PD has not been identified. This gap in our understanding remains a significant barrier to the identification of robust treatment targets for swallowing rehabilitation, and ultimately, long-term health outcomes.
Peripheral muscle weakness is likely one contributing factor to the development of dysphagia in PD. This is supported in part by findings from the limb literature. Researchers have identified that people with PD commonly present with reduced strength of the arms, legs, and trunk, and that these changes in strength are associated with functional impairments in balance, gait, and locomotion [17,18,19]. Over the years, these findings have led to the development of strength training rehabilitation programs for people with PD, which have been found to improve strength and related motor control impairments [20, 21].
Pharyngeal muscle atrophy has been identified as a naturally occurring biological process in healthy older adults (HOA). It is appreciated as an increase in pharyngeal volume, as measured with acoustic pharyngometry, and as increases in pharyngeal area, as measured with fluoroscopy and computed tomography [22,23,24,25]. When present, these pharyngeal anatomic changes can negatively affect swallowing function. HOA with larger pharyngeal cavities demonstrate worse pharyngeal constriction and greater amounts of postswallow residue when compared to HOA with smaller pharyngeal cavities [26]. However, the presence of pharyngeal anatomic changes in PD and their potential impact on swallowing function remain unstudied.
Therefore, the aims of this study were to: (1) determine if changes in pharyngeal area were present in PD above and beyond what is seen during healthy aging by comparing differences in pharyngeal areas between people with PD and HOA; and (2) determine if and how changes in pharyngeal area affect swallowing kinematics, swallowing safety, and swallowing efficiency in PD.
Methods
Participants
A secondary analysis of videofluoroscopic swallow studies (VFSS) was completed comparing HOA and people with PD from two separate prospective studies [26, 27]. All participants signed an informed consent and were treated in accordance with the guidelines and ethical standards of the New York University and the University of Florida Institutional Review Boards (IRB). Approval for the HOA dataset [26] was granted from the New York University IRB (#FY2016-882), and approval for the PD dataset [27] was granted from the University of Florida IRB (#537-2012). Participants from the HOA dataset were recruited from local community centers, while participants from the PD dataset were recruited based on a diagnosis of idiopathic PD. Exclusion criteria for both groups included any history of stroke, head and neck cancer, or respiratory diseases/disorders. Demographic information was recorded for each participant and included the following (as appropriate): age, sex, height, disease duration, l-dopa equivalent dose, Hoehn and Yahr Stage (HYS) [28], and Unified Parkinson’s Disease Rating Scale (UPDRS) [29]. A fellowship-trained movement disorders neurologist made the diagnosis of PD, completed the HYS and UPDRS, and calculated the l-dopa equivalent dose for all participants in the PD group.
Swallowing Evaluations
Healthy Older Adults
The full VFSS protocol for the HOA included 12 self-administered, noncued barium boluses (Varibar; Bracco Imaging); however, only the 5 cc of the noncued ultrathin liquid barium boluses (20% w/v) was included in this analysis. The VF data were collected on a GE Advantix digital fluoroscope (GE Healthcare) at a pulse rate of 30 pulses per second and a capture rate of 30 frames per second (Digital Swallowing Workstation, model 7100, Lincoln Park, NJ: Kay Elemetrics).
Parkinson’s Disease
All VFSS evaluations were completed with participants during the “on” stage of their PD medication (i.e., approximately 1 h after medication). The full VFSS protocol for the PD group included: two 5 cc thin liquid; one self-administered (nonmeasured) thin liquid cup sip; one 90 cc serial liquid cup sip; one 5 cc semisolid by teaspoon; and, a dry solid cracker with barium semisolid for contrast. Forty percent w/v Varibar Barium Sulfate Suspension was used for all liquid boluses, and Varibar Pudding oral paste was used for the semisolid boluses. With the exception of the first 5 cc bolus, all swallows were “noncued” boluses to avoid changes associated with cued swallowing [12, 30]. A high resolution, videofluoroscopic recording device was used for signal acquisition (Digital Swallowing Workstation, model 7100, Lincoln Park, NJ: Kay Elemetrics). Images were captured in the lateral viewing plane, using a continuous image capturing rate of 30 images per second, with a magnification of × 1. Recordings were uploaded into ImageJ software (http://rsb.info.nih.gov/nih-image) for frame-by-frame fluoroscopic analysis.
Outcome Measures
Measurement methodologies for the outcome measures are described below. Pharyngeal area was measured for both the PD and HOA groups in order to examine if differences were present between the two groups. Swallowing kinematics, swallowing efficiency, and swallowing safety were only measured for the PD group in order to determine how changes in pharyngeal area impacted swallowing function.
Pharyngeal Area
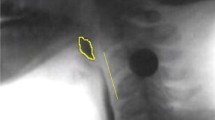
Normalized pharyngeal area was measured using two-dimensional fluoroscopic images and techniques described by Stokey et al. [31] (Fig. 1). This measure involved tracing the pharynx while at rest (i.e., following completion of a 5 cc noncued thin liquid swallow). The area of the pharynx was then normalized to the height of each participant by expressing the pharyngeal area as a percentage of the squared the length from the anterior–inferior points of cervical spine two to cervical spine four (%C2-C42). Normalized pharyngeal area was measured for both the HOA and the PD groups.
Normalized pharyngeal area at rest (PArest): area outlining the pharynx at rest (white line), expressed as a percentage of the squared length of C2–C4 (black, dashed square). This is measured by tracing along the posterior wall from starting from the level of the top of C2 superiorly, and then continuing inferiorly to the piriforms, along the aryepiglottic folds, around the free end of the epiglottis and valleculae, and along the base of tongue
Swallowing Kinematics
Assessment of swallowing kinematics was completed during the 5 cc noncued thin liquid boluses. In total, ten spatial and temporal swallowing kinematics of the pharynx, larynx, and pharyngoesophageal segment (PES) were analyzed, including
- 1.
Peak hyoid position (Hpeak) [32]
- 2.
Peak laryngeal position (Lpeak) [26]
- 3.
Maximal laryngeal constriction area normalized (MLCAn)
- 4.
Maximal pharyngeal constriction area normalized (MPCAn) [31]
- 5.
Maximal PES displacement (PESmax) [33]
- 6.
Onset of hyoid displacement (Honset) [34]
- 7.
Onset of laryngeal vestibule closure (LVConset)
- 8.
Duration of hyoid movement (HMD) [34]
- 9.
Duration of laryngeal vestibule closure (LVCduration) [34]
- 10.
Duration of PES opening (PESduration) [34]
All spatial kinematic measures used the cervical spine as an anatomic scalar to normalize for differences in heights between participants. To measure laryngeal vestibule closure, a novel measurement methodology was developed—“normalized maximal laryngeal constriction area” (MLCAn). MLCAn was obtained by (1) identifying the video frame during maximal laryngeal constriction; and then (2) outlining any unobliterated air space and/or barium contrast visible within the laryngeal vestibule. Like pharyngeal area, MLCAn was then expressed as a percentage of the squared length from C2–C4 (i.e., %C2-C42; Fig. 2).
Maximal laryngeal constriction area normalized (MLCAn): area outlining any unobliterated space within the laryngeal vestibule during the frame of maximal constriction seen during the swallow, expressed as a percentage of the squared length of C2–C4 (%C2-C42). This is measured by outlining any unobliterated airspace or barium contrast within the boundaries of the epiglottic petiole superiorly and anteriorly, the vocal folds inferiorly, and the arytenoids and aryepiglottic folds posteriorly
Swallowing Efficiency
Swallowing efficiency was measured for 5 cc semisolid boluses using the bolus clearance ratio (BCR) [35]. The BCR was measured by first outlining the area of the bolus visible in the pharynx immediately prior to pharyngoesophageal segment (PES) opening, and then outlining the area of the bolus visible in the pharynx immediately following PES closure (Fig. 3). These two measures were then expressed as a ratio to each other. A larger BCR value indicated less bolus clearance (i.e., greater postswallow residue) and therefore, worse swallowing efficiency.
Swallowing Safety
Swallowing safety was captured using the Penetration–Aspiration Scale (PAS) [36]. This 8-point scale was used to characterize the presence of, depth, and reaction to airway invasion. The higher the PAS score, the worse the swallowing safety. The ‘worst PAS’ score identified over the full VFSS protocol was applied in this analysis.
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics for Macintosh, Version 25.0 (ICM Corp, Armonk, NY, USA). A familywise p < 0.05 level was used to determine level of statistical significance.
Reliability
One primary rater blinded to participant demographic information analyzed all measures. Twenty percent of the videos were selected at random for repeat analysis by the primary rater, and one secondary rater, to capture intra- and interrater reliability. Weighted kappas (κW) with linear weights were run to calculate intra- and interrater reliability for PAS. Interpretation for the κW was judged to be ‘excellent’ if ≥ 0.81, ‘good’ if between 0.61 and 0.80, ‘moderate’ if between 0.41 and 0.60, ‘fair’ if between 0.21 and 0.40, and ‘poor’ if < 0.20. Intraclass correlation coefficients (ICC) were run for all other measures, and were judged to be ‘excellent’ if ≥ 0.90, ‘good’ if between 0.75 and 0.90, ‘moderate’ if between 0.50 and 0.75, and ‘poor’ if < 0.50 (Table 1) [37].
Demographics
Descriptive statistics were used to characterize the demographics of the HOA and PD groups. A student t test was used to determine if there were significant differences in ages and heights between the HOA and PD groups.
Aim 1: Compare the Pharyngeal Areas Between People with PD and HOA
An analysis of covariance (ANCOVA) was used to determine if the difference in pharyngeal areas between the HOA and PD groups was significantly different, while controlling for age, sex, and height.
Aim 2: Assess the Influence of Pharyngeal Area on Swallowing Kinematics in PD
A multivariate regression was used to assess the influence of pharyngeal area on swallowing kinematics in PD. A canonical correlation was used as a multivariate post hoc analysis to describe the combination of changes in swallowing kinematics. Simple linear regressions were used as univariate post hoc analyses to determine the influence of pharyngeal area on each kinematic variable in isolation.
Aim 3: Assess the Influence of Pharyngeal Area on Swallowing Efficiency and Safety in PD
A simple univariate regression was used to determine the influence of pharyngeal area on swallowing efficiency (BCR) in PD, while controlling for age and sex. In addition, an ordinal logistic regression was used to determine the influence of pharyngeal area on swallowing safety (PAS) in PD, while controlling for age and sex.
Results
Demographics
Demographic information for the HOA and PD groups are outlined in Table 2. The HOA group (n = 41, 19 males) had a mean age of 76.4 years (± 7.2), and a mean height of 164.9 cm (± 9.5). The PD group (n = 40, 29 males) had a mean age of 63.2 years (± 8.9), a mean height of 173.4 cm (± 9.9), a mean disease duration of 8.0 years (± 3.9), a median HYS of II, and a mean UPDRS of 28.8 (± 10.0) There was a statistically significant difference in mean height between the two groups, t(79) = − 3.993, p < .0005. There was also a statistically significant difference in mean age between the two groups, t(79) = 7.302, p < .0005.
Aim 1: Compare the Pharyngeal Areas Between People with PD and HOA
The HOA group had a mean PArest of 62.4 %C2-C42 (± 20.3), and the PD group had a mean PArest of 72.3 %C2-C42 (± 25.0). PArest was significantly larger for the PD group when compared to the HOA group F(1, 22.552) = 8.506, p = .008, adjusted R2 = .274, observed power = .797. After controlling for age, sex, and height, the PD group demonstrated an average PArest that was 21.1% larger than the HOA group.
Aim 2: Assess the Influence of Pharyngeal Area on Swallowing Kinematics in PD
Descriptive statistics of the swallowing kinematics are outlined in Table 3. Multivariate regression revealed that pharyngeal area significantly influenced swallowing kinematics in PD, F(11, 28) = 4.686, p = .001, Wilks’ Λ = .374 (Table 4). Post-hoc multivariate analysis revealed that an increase in pharyngeal area was associated with a combination of changes including reduced duration of airway closure (LVCduration) (r = − .488), decreased pharyngeal constriction (MPCAn) (r = .470), decreased laryngeal constriction (MLCAn) (r = .278), increased extent of hyoid displacement (Hpeak) (r = .299), increased pharyngeal esophageal segment opening (PESmax) (r = .372), and prolonged duration of hyoid movement (HMD) (r = .290) (Table 4). Post-hoc univariate analysis revealed that an increase in pharyngeal area was associated with significantly reduced pharyngeal constriction (r = .352) and duration of airway closure (r = − .351).
Aim 3: Assess the Influence of Pharyngeal Area on Swallowing Efficiency and Safety in PD
Descriptive statistics of the BCR and PAS are outlined in Table 3. Simple linear regression revealed that pharyngeal area significantly influenced swallowing efficiency in PD, F(3, 34) = 3.162, p = .037, adjusted R2 = .218. As pharyngeal area increased, swallowing efficiency decreased (i.e., there were greater amounts of postswallow residue). In addition, the ordinal logistic regression also revealed that pharyngeal area significantly influenced swallowing safety in PD, χ2(32) = 72.818, p < .0005. An increase in pharyngeal area was associated with worse swallowing safety.
Discussion
This study compared the pharyngeal areas of HOA to people with PD, and examined the influence of pharyngeal area on swallowing function in PD. Increases in pharyngeal area can occur as a function of healthy normal aging, and is thought to be the consequence age-related sarcopenia and muscle atrophy. The results from this study revealed that, on average, people with PD exhibited larger pharyngeal areas when compared to HOA and that these changes in pharyngeal area were significantly associated with maladaptive changes to swallowing kinematics and worse swallowing safety and efficiency.
The people with PD in this study demonstrated larger pharyngeal areas when compared to published norms in healthy young adults [38], and when compared to the normative HOA group in this study. The presence of larger pharyngeal areas in this PD cohort suggest that pharyngeal muscle atrophy may be present in PD above and beyond what is seen in normal healthy aging, and that these changes may be contributing factors to the development of dysphagia. This hypothesis is supported in part by research from Mu et al., who completed histological studies examining the effects of PD on postmortem pharyngeal anatomy. These histological studies revealed that postmortem PD pharynges exhibit significantly more atrophic muscle fibers and motor neuron degeneration compared to the pharynges of age-matched controls. Furthermore, Mu and colleagues found that the magnitude of pharyngeal muscle atrophy and motor neuron degeneration was greater in the dysphagic postmortem PD pharynges when compared to the non-dysphagic postmortem PD pharynges [39, 40]. However, because direct diagnostic testing of muscle composition was not completed the present study, the presence of sarcopenia and pharyngeal atrophy cannot be conclusively determined. Future work including needle biopsy and VFSS would be required in order to more objectively determine the presence of pharyngeal muscle atrophy in PD and its impact on swallowing function.
This study also revealed that an increase in pharyngeal area was associated with detrimental changes to swallowing kinematics, swallowing safety, and swallowing efficiency in PD. Specifically, as pharyngeal area increased, the extent of pharyngeal constriction and duration of airway closure decreased. The average pharyngeal constriction in the PD group from this study was approximately 7.5 times greater (i.e., worse) as compared to published norms in healthy older adults [26]. In addition, the average duration of laryngeal vestibule closure (0.43 s) also appeared reduced in this PD group as compared to published norms in healthy older adults (~ 0.50 s) [41,42,43]. Pharyngeal constriction and duration of airway closure are crucial for safely and efficiently transporting foods and liquids through the pharynx while preventing the aspiration of ingested boluses into the lungs. In fact, these swallowing kinematic variables have been identified as statistically significant predictors of residue and airway invasion in PD [12].
It should be noted that both spatial and temporal swallowing kinematics were influenced by differences in pharyngeal area, and presumably, pharyngeal muscle weakness. This is of significant clinical interest given that “muscle weakness” is often associated with changes to spatial kinematics (e.g., reduced extent of hyoid displacement), while “sensory” and “coordination” impairments are often associated with changes to temporal swallowing kinematics (e.g., onset of pharyngeal swallow initiation). Clinicians interpreting VFSS should be aware that strength-based impairments may impact both spatial and temporal swallowing kinematics when attempting to identify the potential causes of dysphagia and the types of rehabilitation exercises that may be most appropriate.
Findings from this study contribute to a growing understanding of the pathophysiologies that may contribute to dysphagia in PD, and thus, a growing understanding of the potential therapeutic approaches that may be efficacious for improving swallowing dysfunction in PD. Because motor control and coordination impairments are thought to be the primary source for swallowing dysfunction in PD due to the pathology of the disease, “skill-based” swallowing interventions have become an increasingly popular intervention when rehabilitating individuals with movement disorders [44,45,46]. However, given that the findings from the present study suggest that pharyngeal muscle atrophy may also be contributing to PD-related dysphagia, “strength-based” exercises may be of additional benefit for rehabilitating this patient population. We hypothesize that resistance training aimed at maintaining or increasing pharyngeal muscle strength may be beneficial at managing changes to spatial kinematics (e.g., pharyngeal constriction, laryngeal constriction), temporal kinematics (e.g., duration of laryngeal vestibule closure), swallowing safety, and swallowing efficiency.
This study is not without limitations. One limitation is the use of two separate datasets that had significant differences in height and age. The PD group was an average of 8 cm taller than the HOA group which may have contributed to the PD group having larger pharyngeal areas. However, this difference in heights between the two groups was controlled by: (1) using a statistical model that controlled for height differences (ANCOVA); and (2) using a measurement methodology that accounted for height differences with use of an anatomic scalar [32]. There was also a significant difference in age between the two groups, with the HOA group being an average of 10 years older than the PD group. This difference was also controlled for statistically. However, given that aging has been found to increase pharyngeal volume, one would have expected the HOA group to have larger pharyngeal areas compared with the younger PD group. The fact that pharyngeal area for the PD group was larger despite their being younger further strengthens the conclusion that PD significantly affects pharyngeal size. A second potential limitation to this study is that the PD group was evaluated during the “on” stage of their PD medications. While people with PD are traditionally evaluated during the “on” stage and several studies have identified no changes to pharyngeal swallowing function while ‘on’ Levodopa, we cannot discount the possibility that this may have influenced the findings related to the influence of pharyngeal cavity size on swallowing function.
Clinical Implications
This study identified that people with PD exhibit larger pharyngeal areas when compared to their healthy adult peers. These changes in pharyngeal area may significantly affect swallowing kinematics and contribute to the presence of residue, penetration, and aspiration. Clinicians should attend to pharyngeal area during videofluoroscopy, consider the impact that pharyngeal muscle atrophy may have in contributing to a patient’s dysphagia, and integrate pharyngeal resistance training exercises aimed to manage pharyngeal muscle weakness in PD.
References
Kalf JG, De Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Park Relat Disord. 2012;18:311–5. https://doi.org/10.1016/j.parkreldis.2011.11.006.
Altman KW, Yu G-P, Schaefer SD. Consequence of dysphagia in the hospitalized patient impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136:784–9.
Pikus L, Levine MS, Yang YX, et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. Am J Roentgenol. 2003;180:1–4. https://doi.org/10.2214/ajr.180.6.1801613.
Fall P, Saleh A, Fredrickson M, Olsson J, Granerus A. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease. Mov Disord. 2003;18(11):1312–6.
Hegland KW, Troche MS, Brandimore A. Relationship between respiratory sensory perception, speech, and swallow in Parkinson’s disease. Mov Disord Clin Pract. 2019;6(3):243–9. https://doi.org/10.1002/mdc3.12732.
Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and Parkinson’s disease. Dysphagia. 2012;27:106–14. https://doi.org/10.1007/s00455-011-9347-z.
Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in parkinson disease. Chest. 2014;146(5):106. https://doi.org/10.1378/chest.14-0066.
Troche MS, Brandimore AE, Hegland KW, Zeilman PR, Foote KD, Okun MS. Tailored deep brain stimulation optimization for improved airway protective outcomes in Parkinson’s disease. Interdiscip Neurosurg. 2016;5:3–5. https://doi.org/10.1016/j.inat.2016.03.003.
Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson’s disease: a systematic review. Park Relat Disord. 2013;19:783–8. https://doi.org/10.1016/j.parkreldis.2013.05.001.
Warnecke T, Suttrup I, Schröder JB, et al. Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Park Relat Disord. 2016. https://doi.org/10.1016/j.parkreldis.2016.04.034.
Sutton JP. Dysphagia in Parkinson’s disease is responsive to levodopa. Park Relat Disord. 2013. https://doi.org/10.1016/j.parkreldis.2012.11.007.
Curtis JA, Molfenter S, Troche MS. Predictors of residue and airway invasion in Parkinson’s disease. Dysphagia. 2011. https://doi.org/10.1007/s00455-019-10014-z.
Gao J, Guan X, Cen Z, et al. Alteration of brain functional connectivity in Parkinson’s disease patients with dysphagia. Dysphagia. 2019. https://doi.org/10.1007/s00455-019-10015-y.
Perry SE, Sevitz J, Curtis JA, Troche MS. The influence of tongue strength on oral bolus preparation, swallowing efficency, and airway invasion in Parkinson’s disease. In: 26th Annual Meeting of the Dysphagia Research Society, Baltimore. 2018.
Troche M, Okun M, Rosenbek J, et al. Aspiration and swallowing in Parkinson’s disease and rehabilitation with EMST: a randomized trial. Mov Disord. 2010;75:1912–9.
Pitts LL, Morales S, Stierwalt JAG. Lingual pressure as a clinical indicator of swallowing function in Parkinson’s disease. J Speech Lang Hear Res. 2018;61(2):257–65. https://doi.org/10.1044/2017_JSLHR-S-17-0259.
Schilling BK, Karlage RE, LeDoux MS, Pfeiffer RF, Weiss LW, Falvo MJ. Impaired leg extensor strength in individuals with Parkinson disease and relatedness to functional mobility. Park Relat Disord. 2009. https://doi.org/10.1016/j.parkreldis.2009.06.002.
Nallegowda M, Singh U, Handa G, et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson’s disease: a pilot study. Am J Phys Med Rehabil. 2004;8(12):898–908. https://doi.org/10.1097/01.PHM.0000146505.18244.43.
Scandalis TA, Bosak A, Berliner JC, Helman LL, Wells MR. Resistance training and gait function in patients with Parkinson’s disease. Am J Phys Med Rehabil. 2001;80(1):38–43. https://doi.org/10.1097/00002060-200101000-00011.
Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait Posture. 2012;35(4):669–73. https://doi.org/10.1016/j.gaitpost.2011.12.022.
Ni M, Signorile JF, Balachandran A, Potiaumpai M. Power training induced change in bradykinesia and muscle power in Parkinson’s disease. Park Relat Disord. 2016;23:37–44. https://doi.org/10.1016/j.parkreldis.2015.11.028.
Molfenter SM, Amin MR, Branski RC, et al. Age-related changes in pharyngeal lumen size: a retrospective MRI analysis. Dysphagia. 2015;30(3):321–7. https://doi.org/10.1007/s00455-015-9602-9.
Aminpour S, Leonard R, Fuller SC, Belafsky PC. Pharyngeal wall differences between normal younger and older adults. Ear Nose Throat J. 2011;90(4):E1–5.
Leonard R, Kendall KA, Mckenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19:133–41. https://doi.org/10.1007/s00455-003-0508-6.
Inamoto Y, Saitoh E, Okada S, et al. Anatomy of the larynx and pharynx: effects of age, gender and height revealed by multidetector computed tomography. J Oral Rehabil. 2015;42(9):670–7. https://doi.org/10.1111/joor.12298.
Molfenter SM, Lenell C, Lazarus CL. Volumetric changes to the pharynx in healthy aging: consequence for pharyngeal swallow mechanics and function. Dysphagia. 2019;34(1):129–37. https://doi.org/10.1007/s00455-018-9924-5(.
Troche MS, Schumann B, Brandimore AE, Okun MS, Hegland KW. Reflex cough and disease duration as predictors of swallowing dysfunction in Parkinson’s disease. Dysphagia. 2016;31:757–64. https://doi.org/10.1007/s00455-016-9734-6.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427–442. http://www.neurology.org/content/17/5/427.citation.
Disease MDSTF on RS for P. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–750. https://doi.org/10.1002/mds.10473.
Daniels SK, Schroeder MF, Degeorge PC, Corey DM, Rosenbek JC. Effects of verbal cue on bolus flow during swallowing. Am J Speech-Lang Pathol. 2007;16:140–7. https://doi.org/10.1044/1058-0360(2007/018).
Stokely SL, Peladeau-Pigeon M, Leigh C, Molfenter SM, Steele CM. The relationship between pharyngeal constriction and post-swallow residue. Dysphagia. 2015;30:349–56. https://doi.org/10.1007/s00455-015-9606-5.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid Excursion during swallowing. J Speech Lang Hear Res. 2014;57:768–78. https://doi.org/10.1044/2014_JSLHR-S-13-0152.
Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(3):146–52. https://doi.org/10.1007/s004550010017.
Molfenter SM, Steele CM. Variation in temporal measures of swallowing: sex and volume effects. Dysphagia. 2013;28:226–33. https://doi.org/10.1007/s00455-012-9437-6.
Leonard R. Two Methods for Quantifying Pharyngeal Residue on Fluoroscopic Swallow Studies: reliability Assessment. Ann Otolaryngolg Rhinol. 2017;4(3):1–5.
Rosenbek JC, Robbins J, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Steele CM, Peladeau-Pigeon M, Barbon CAE, et al. Reference values for healthy swallowing across the range from thin to extremely thick liquids. J Speech Lang Hear Res. 2019. https://doi.org/10.1044/2019_jslhr-s-18-0448.
Mu L, Sobotka S, Chen J, et al. Altered pharyngeal muscles in parkinson disease. J Neuropathol Exp Neurol. 2012;71(6):520–30. https://doi.org/10.1097/NEN.0b013e318258381b.
Mu L, Sobotka S, Chen J, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral. J Neuropathol Exp Neurol. 2013;72(2):119–29.
Kurosu A, Logemann JA. Gender effects on airway closure in normal subjects. Dysphagia. 2010;25(4):284–90. https://doi.org/10.1007/s00455-009-9257-5.
Humbert IA, Sunday KL, Karagiorgos E, et al. Swallowing kinematic differences across frozen, mixed, and ultrathin liquid boluses in healthy adults: age, sex, and normal variability. J Speech Lang Hear Res. 2018;1:1. https://doi.org/10.1044/2018_jslhr-s-17-0417.
Molfenter SM, Steele CM. Temporal variability in the deglutition literature. Dysphagia. 2012;27(2):162–77. https://doi.org/10.1007/s00455-012-9397-x.
Perry SE, Sevitz JS, Curtis JA, Kuo S-H, Troche MS. Skill training resulted in improved swallowing in a person with multiple system atrophy: an endoscopy study. Mov Disord Clin Pract. 2018;4(5):451–2. https://doi.org/10.1002/mdc3.12628.
Athukorala RP, Jones RD, Sella O, Huckabee M-L. Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Arch Phys Med Rehabil. 2014;95(7):1374–82. https://doi.org/10.1016/j.apmr.2014.03.001.
Manor Y, Mootanah R, Freud D, Giladi N, Cohen JT. Video-assisted swallowing therapy for patients with Parkinson’s disease. Park Relat Disord. 2013. https://doi.org/10.1016/j.parkreldis.2012.10.004.
Acknowledgements
This work was supported in part by an NIH (NCATS) CTSA through the University of Florida (UL1TR000064 and KL2TR000065), awarded to Dr. Michelle S. Troche, and also in part by the NIH National Institute of Deafness and Other Communication Disorders (1R21DC015067), awarded to Dr. Sonja M. Molfenter.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors James A. Curtis, Sonja M. Molfenter, and Michelle S. Troche declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Curtis, J.A., Molfenter, S.M. & Troche, M.S. Pharyngeal Area Changes in Parkinson’s Disease and Its Effect on Swallowing Safety, Efficiency, and Kinematics. Dysphagia 35, 389–398 (2020). https://doi.org/10.1007/s00455-019-10052-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-019-10052-7