Abstract
Dysphagia is a common non-primary symptom of patients with Parkinson’s disease. The aim of this study is to investigate the underlying alterations of brain functional connectivity in Parkinson’s disease patients with dysphagia by resting-state functional magnetic resonance imaging. We recruited 13 Parkinson’s disease patients with dysphagia and ten patients without dysphagia, diagnosed by videofluoroscopic study of swallowing. Another 13 age and sex-matched healthy subjects were recruited. Eigenvector centrality mapping was computed to identify functional connectivity alterations among these groups. Parkinson’s disease patients with dysphagia had significantly increased functional connectivity in the cerebellum, left premotor cortex, the supplementary motor area, the primary motor cortex, right temporal pole of superior temporal gyrus, inferior frontal gyrus, anterior cingulate cortex and insula, compared with patients without dysphagia. This study suggests that functional connectivity changes in swallowing-related cortexes might contribute to the occurrence of dysphagia in Parkinson’s disease patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is one of the most common gastrointestinal symptoms in Parkinson’s disease (PD) patients, of which the incidence is up to 95% [1]. The typical symptoms of dysphagia are chewing difficulties, leakage of food, prolonged swallowing time, which can develop into malnutrition, aspiration pneumonia and other serious problems. In view of the different manifestations of dysphagia, many patients suffer from dysphagia without self-knowledge. As the gold standard for the diagnosis of dysphagia [2], videofluoroscopic study of swallowing (VFSS) can discover invisible aspiration and leakage, and provide guidance for rehabilitation treatment of dysphagia. However, the pathophysiology underlying dysphagia in PD patients is still poorly understood [3, 4].
There have been several imaging studies on dysphagia in PD. Akio Kikuchi et al. found that compared with the normal subjects, the glucose metabolism in the supplementary motor areas (SMA) and the anterior cingulate cortex (ACC) decreased in PD patients with dysphagia by 18F-fluorodeoxyglucose positron emission tomography (PET) [5]. This suggested that the dysfunction of the SMA region might be associated with impaired swallowing initiation in PD patients with dysphagia, meanwhile the impairment of middle and caudal regions of the ACC may contribute to the active swallowing process. In addition, a magnetoencephalography (MEG) study demonstrated that cortical swallowing processing in PD patients without dysphagia was characterized by a pronounced shift of peak activation toward lateral parts of the premotor, motor, and inferolateral parietal cortex with reduced activation of the SMA, suggesting that the compensatory mechanisms in the cerebral cortex of PD patients without dysphagia may help prevent swallowing impairment [6]. However, because the number of previous studies was small, we are still far away from fully understanding functional alterations of the brain in PD with dysphagia.
Resting-state functional magnetic resonance imaging (rsfMRI) is an important method for studying brain function because of its high temporal and spatial resolution and non-invasiveness. Eigenvector centrality mapping (ECM) can automatically analyze the whole brain data and identify brain regions as a function of the central link, to avoid studying bias caused by the selection of region of interest [7, 8]. In order to further explore the relationship between brain functional network and pathophysiological mechanisms in PD patients with dysphagia, we intend to use ECM to analyze the whole brain functional connectivity, and compare the differences among PD patients with dysphagia, PD patients without dysphagia and normal controls (NCs).
Patients and Methods
Subjects
Twenty-three patients (13 dysphagic and ten non-dysphagic patients) diagnosed with Parkinson’s disease according to the UK Parkinson’s disease brain bank criteria, as well as 13 age and sex-matched NCs were recruited for this study. Exclusion criteria included: (1) history of other diseases affecting swallowing function; (2) pneumonia at the time of enrolment, renal and cardiac insufficiency; (3) Kubota water drinking test grade > 3; (4) indwelling nasogastric tube or gastrostomy; (5) metal clips in the brain; or (6) patients with mental disorders or dementia and uncooperative patients [9, 10]. Of note, Kubota water drinking test is frequently used in clinical practice for dysphagia evaluation. Patient is to sit upright and asked to drink 30 ml of warm water while time taken and number of coughs are recorded. Grade 1 (excellent): able to swallow water in a single try without coughing. Grade 2 (good): able to swallow water in two tries without coughing. Grade 3 (average): able to swallow in a single try but with coughing. Grade 4 (fair): takes two or more tries to swallow and with coughing. Grade 5 (poor): repeated coughing, able to swallow some but not all of the water. Each participant signed the informed written consent, and the study was approved by the Medical Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Dysphagia Diagnosis and Clinical Assessments
All VFSS examinations were done in the “off” state (all PD patients stopped taking anti-PD drugs for more than 12 h before the examination). The measurements were evaluated and recorded, including oral transit time (OTT), pharyngeal transit time (PTT), pharyngeal delay time (PDT), upper esophageal sphincter opening duration (UESod), residue in the pharyngeal cavity, and Penetration/aspiration Scale (PAS). Those who displayed at least one of the following symptoms were diagnosed as dysphagia: OTT > 1.5 s, PTT > 1.0 s, PDT > (< 60 years old. 0.24 s: > 60 years old, 0.36 s), UESod > 0.51 s, residue, PAS > 2 [11] (Supplementary Table). The procedure of VFSS and the diagnostic criteria of dysphagia were based on the ones we previously used [11]. 36 subjects detailed records of medical history, disease course and demographic information were collected. A detailed neurological examination was performed on all PD patients, including the Unified Parkinson’s Disease Rating Scale, Part III (UPDRS-III), Hoehn and Yahr Scale and Mini-Mental State Evaluation (MMSE).
rsfMRI Data Acquisition
Each subject (PD patients in the “off” state) was scanned on a 3.0T GE SIGNA MR scanner (GE Healthcare Wauwatosa, WI) in the Department of Radiology of the Second Affiliated Hospital of Zhejiang University School of Medicine. To respectively reduce noise and head motion, ear plugs and foam pads were used. Subjects were instructed to relax with their eyes closed and avoid falling asleep. Brain abnormalities were first screened using T1-and T2-weighted images to further exclude unqualified subjects. To acquire blood-oxygenation-level-dependent (BOLD) images, parameters used in echo planar imaging sequence was as follows: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 240 × 240 mm2, matrix = 64 × 64, slice thickness = 5 mm, slice gap = 1 mm and slice number = 23. A total of 185 resting-state BOLD images were collected from each subject. T1-weighted anatomical images, acquired after BOLD images, were scanned using the following parameters: TR = 5.14 ms, TE = 1.17 ms, flip angle = 13°, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1 mm3 and slice number = 124.
rsfMRI preprocessing
SPM8 (Statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm) was used to perform the standard preprocessing of rsfMRI data including first ten time points removal, layer time correction, head motion correction, brain normalization to EPI template in the MNI space, spatial smoothness with Gauss kernel with a full width and half height of 6 mm, 0.01–0.08 Hz band-pass filter and linear detrending.
ECM Computation
The computational method of fast ECM (https://code.google.com/p/bias/source/browse/matlab/fastECM), as one of graph theory analysis, was used to calculate the center degree of the eigenvector, generating a voxel-wise measure of relevance to the functional brain network, which was demonstrated having more efficiency than the traditional method of functional connectivity computation [12]. Biologically, eigenvector centrality maps can reflect the global influence of each voxel in the brain network.
Statistical Analysis
For testing the distribution of clinical data, including age, sex, course of disease, MMSE score, Hoehn and Yahr scale, and UPDRS-III score, SPSS 25 (IBM, USA) statistical software was used for analysis and processing. To compare these clinical data among the PD patients with dysphagia, PD patients without dysphagia and NCs, One-way analysis of variance, Chi square test, t test and Mann–Whitney U analysis were performed appropriately depending on the Gaussian distribution. P < 0.05 was regarded as statistically significant.
For the imaging data, we used the two-sample t test based on the random effect analysis in the SPM8 software, and compared the statistical brain maps of the center degree of the eigenvectors among the three groups. The final results were displayed on the standard MNI 152 T1 template. The setting threshold was P < 0.05, cluster size = 228 (= P < 0.05, corrected).
Results
Demographic Characteristics and Clinical Information
There was no significant difference in gender, age, disease duration, MMSE score, Hoehn and Yahr scale and UPDRS-III score between PD patients with dysphagia and PD patients without dysphagia. In addition, the age and sex of NCs were not significantly different from those of the other two groups. (Table 1).
Comparison of Functional Connectivity Between PD Patients with Dysphagia and PD Patients Without Dysphagia
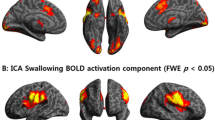
Compared with PD patients without dysphagia, PD patients with dysphagia showed enhanced functional connectivity in the left cerebellar tonsil, cerebellum 8 region, precentral gyrus, postcentral gyrus, premotor cortex, supplementary motor area, the primary motor cortex, right superior temporal gyrus, temporal inferior frontal gyrus, middle frontal gyrus, anterior cingulate, orbitofrontal gyrus with the cortex, insula and bilateral middle frontal gyrus (Fig. 1, Table 2).
Statistical parametric map showing the significant differences in functional connectivity among three groups: a differences between PD with dysphagia and PD without dysphagia, b differences between PD without dysphagia and NCs; c differences between PD with dysphagia and NCs. Compared to the latter group, areas in red indicate regions in which the former group had increased functional connectivity, while in blue indicate regions in which the former group had decreased functional connectivity. The threshold for display was set to P < 0.05
Comparison of Functional Connectivity Between PD Patients Without Dysphagia and NCs
Compared with the NCs, the functional connectivity of the right insula, inferior frontal gyrus, inferior frontal lobe, putamen and globus pallidus was decreased in the PD patients without dysphagia. (Figure 1, Table 3).
Comparison of Functional Connectivity Between PD Patients with Dysphagia and NCs
In PD patients with dysphagia, the functional connectivity of left cerebellar tonsil, cerebellum eight region, cerebellum nine region and spindle gyrus was increased, compared with NCs. (Figure 1, Table 4).
Discussion
To the best of our knowledge, this is the first study to explore the alterations of brain functional connectivity in PD patients with dysphagia diagnosed by VFSS, using the rsfMRI technique. The most important finding of our research was that PD patients with dysphagia had significantly increased functional connectivity mainly in the left cerebellum, premotor cortex, SMA, the primary motor cortex, and right temporal gyrus, inferior frontal gyrus, ACC and insula, compared with PD patients without dysphagia.
The brain regions we found in this study were consistent with previous studies. In Hamdy et al. first found that the sensorimotor cortex, inferior frontal gyrus, insula, temporal lobe, cerebellum and brain stem were involved in the process of voluntary swallowing while exploring the fluorodeoxyglucose metabolism using PET [13]. There was little agreement regarding activation in the cerebellum [14]. Our findings demonstrated that the cerebellum, premotor cortex, SMA, the primary motor cortex, temporal gyrus, inferior frontal gyrus, ACC and insula, played important roles in swallowing.
Nevertheless, how these brain regions functionally changed in PD patients with dysphagia remained to be uncertain. In 2009, Li et al. investigated the task-state fMRI images of patients with dysphagia after stroke and found enhanced brain activations in PD patients with dysphagia, compared with the NCs [15]. Liu et al. found in a meta-analysis that stroke patients with dysphagia showed hyperactivation in left cingulate gyrus, left precentral gyrus and right posterior cingulate gyrus, and hypoactivation in right cuneus and left middle frontal gyrus, when compared with NCs by fMRI [16]. Suntrup et al. examined the cortical representation of volitional swallowing by MEG, and found different activations in PD patients with or without dysphagia [6]. They assumed that there might be compensatory pathways which were no longer recruitable in dysphagic patients due to the degeneration of neurons. However, in a PET study, Kikuchi et al. observed in another PET study that compared with NCs, PD patients with dysphagia showed decreased glucose metabolism in SMA and ACC areas [5]. It was not consistent to our finding of enhanced functional connectivity in these regions. Possibly, different physiological mechanisms between PET and fMRI were indicated. It was worth noting that the cerebellum, which was rarely discussed in the previous literature, showed significantly increased functional connectivity in PD patients with dysphagia. In 2001, Mosier and Bereznaya proposed that parallel cortical networks may exist for volitional control of swallowing, and the cerebellum has inhibitory connections to the primary motor cortex, primary sensory cortex, SMA, and cingulate cortex [17]. It has been mentioned that both hemispheres of cerebellum participate in healthy swallowing, which may contribute to specific physiological functions of swallowing, and, through repetitive transcranial magnetic stimulation in the cerebellum. Vasant et al. found the pharyngeal corticobulbar excitability was modulated with long-lasting effects. [18]. [19]. Similarly, in the present study, the enhanced functional connectivity in the cerebellum further demonstrated its important role in swallowing pathway, which might be over-compensated in PD status. With the fMRI results gathered during resting state, we can deduce that in PD patients with dysphagia, excitability of swallowing-related cortexes was increased in the basic state, like higher “threshold”, which led to relatively lower activation caused by the same food stimulation in the process of swallowing, compared with that in PD patients without dysphagia. In the meanwhile, the cerebellum helps regulates the compensatory swallowing mechanism. This may explain the dysphagic symptoms such as deglutition initial delay, esophageal sphincter dysfunction, etc. To test these hypotheses, longitudinal study is warranted to observe the progression of PD patients without dysphagia, and detect the functional connectivity which is expected to be enhanced in the patients who may potentially develop dysphagia.
Another finding of this study was that compared with the NCs, the functional connectivity of the right insula, inferior frontal gyrus, inferior frontal lobe, putamen and globus pallidus was decreased in PD patients without dysphagia. According to the literature, the volume of putamen, caudate nucleus and globus pallidus was decreased in PD patients [20], and the expression of dopamine transporter protein was also decreased [21]. There were three studies conducting whole brain ECM in PD patients. Lou et al. found that compared to NCs, non-depressed PD patients showed significantly decreased functional connectivity in the temporal gyrus, frontal gyrus, postcentral gyrus, orbital gyrus, superior parietal lobule, occipital gyrus, precuneus and insula [22]. In a study done by Schipper et al., decreased functional connectivity in PD patients was found in occipital and frontal parts of the brain, compared to control subjects [23]. Mueller et al. investigated ECM changes in PD patients after deep brain stimulation and levodopa treatment, and reported that functional connectivity was increased in the brainstem, which was related to motor improvement [24]. Connectivity reductions of frontal and occipital regions were reported as well in some rsfMRI studies using other techniques other than ECM in PD [25, 26]. Our study showed that functional connectivity in the right insula, inferior frontal gyrus, inferior frontal lobe, putamen and globus pallidus, was decreased in PD patients without dysphagia, consistent with previous reports. We think it may be related to the pathophysiology of PD, which was based on the loss of massive degeneration of dopaminergic neurons in the dense part of the substantia nigra, resulting in clinical symptoms. The projective fibers of the substantia nigra were mainly confined to the caudate nucleus and the putamen. Therefore, the decrease in dopamine content in PD patients can lead to structural and functional abnormalities in the corresponding parts.
Clinical evaluation of dysphagia today relies on self-report, bed-side scale and Kubota water drinking test. The accuracy of these methods is not satisfactory. VFSS and fiberoptic endoscopy are required for precise diagnosis. VFSS has the risk of radiation and aspiration, while fiberoptic endoscopy is an invasive procedure. RsfMRI used in this study is safe, convenient, and effective, which may be helpful for clinical diagnosis and early intervention in PD patients with dysphagia. Further rsfMRI studies in PD patients with dysphagia are needed.
There are limitations of this study: 1. For the safety of the patients, the study excluded the ones whose Kubota water drinking test grade were over 3, to avoid aspiration in the examination, which may result in the absence of patients with severe dysphagia; 2. The sample size of this study was small. In view of the high incidence of dysphagia in the PD patients, the number of patients without dysphagia was particularly limited, leading to the possibility of bias. Increasing the sample size to further verify the results of our study can increase the reliability of the conclusions; 3. The control group was not confirmed by VFSS examination, so the effect of probable dysphagia among NCs could not be completely eliminated; 4. The medication load was not well matched between the two groups of PD patients. Therefore, to maximally eliminate the influence of medication, the clinical evaluation and MRI scanning were performed after withdrawing the anti-PD drugs for at least 12 h in PD patients.
Conclusion
Dysphagia, as a common complication of PD, has a varying degree of impact on the quality of life in PD patients. This rsfMRI study, found that the enhanced functional connectivity of the premotor area, SMA, primary motor area, ACC, and insular lobe might be closely related to the pathophysiological mechanism of dysphagia in PD patients.
References
Pflug C, Bihler M, Emich K. Critical dysphagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia. 2018;33(1):41–50.
Costa MM. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq Gastroenterol. 2010;47(4):327–8.
Michou E, Hamdy S. Dysphagia in Parkinson’s disease: a therapeutic challenge? Expert Rev Neurother. 2010;10(6):875–8.
Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dysphagia. 2016;31(1):24–32.
Kikuchi A, Baba T, Hasegawa T, et al. Hypometabolism in the supplementary and anterior cingulate cortices is related to dysphagia in Parkinson’s disease: a cross-sectional and 3-year longitudinal cohort study. BMJ Open. 2013;3(3):e002249.
Suntrup S, Teismann I, Bejer J, et al. Evidence for adaptive cortical changes in swallowing in Parkinson’s disease. Brain. 2013;136(3):726–38.
Lohmann G, Margulies DS, Horstmann A, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS ONE. 2010;5(4):e10232.
Jech R, Mueller K, Schroeter ML, et al. Levodopa increases functional connectivity in the cerebellum and brainstem in Parkinson’s disease. Brain. 2013;136(7):e234.
Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–37.
Katzman R, Zhang MY, Ouang-Ya-Qu, et al. A chinese version of the mini-mental state examination; impact of illiteracy in a shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971–8.
Ding X, Gao J, Xie C, et al. Prevalance and clinical correlation of dysphagia in Parkinson disease: a study on Chinese patients. Eur J Clin Nutr. 2018;72(1):82–6.
Wink AM, de Munck JC, van der Werf YD, et al. Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect. 2012;2(5):265–74.
Hamdy S, Rothwell J, Brooks D, et al. Identification of the cerebral loci processing human swallowing with H2(15) O PET activation. J Neurophysiol. 1999;81(4):1917–26.
Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22(3):266–75.
Li S, Luo C, Yu B, et al. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry. 2009;80(12):1320–9.
Liu L, Xiao Y, Zhang W, et al. Functional changes of neural circuits in stroke patients with dysphagia: a meta-analysis. J Evid Based Med. 2017;10(3):189–95.
Mosier K, Bereznaya I. Parallel cortical networks for voli- tional control of swallowing in humans. Exp Brain Res. 2001;140:280–9.
Rangarathnam B, Kamarunas E, McCullough GH. Role of cerebellum in deglutition and deglutition disorders. Cerebellum. 2014;13(6):767–76.
Vasant DH, Michou E, Mistry S, et al. High-frequency focal repetitive cerebellar stimulation induces prolonged increases in human pharyngeal motor cortex excitability. J Physiol. 2015;593(22):4963–77.
Geng D, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006;58(2):256–62.
Wang J, Jiang YP, Xiang JD, et al. The significance of 18F-FP- CIT dopamine transporter PET imaging in early diagnosis of Parkinson’s disease. Chin J Nucl Med. 2003;23(4):216–8.
Lou Y, Huang P, Li D, et al. Altered brain network centrality in depressed Parkinson’s disease patients. Mov Disord. 2015;30(13):1777–84.
de Schipper LJ, Hafkemeijer A, van der Grond J, et al. Altered whole-brain and network-based functional connectivity in Parkinson’s disease. Front Neurol. 2018;9:419.
Mueller K, Jech R, Růžička F, Holiga Š, et al. Brain connectivity changes when comparing effects of subthalamic deep brain stimulation with levodopa treatment in Parkinson’s disease. Neuroimage Clin. 2018;19:1025–35.
Lewis SJG, Dove A, Robbins TW, et al. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6.
Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–9.
Acknowledgments
The authors thank all the Parkinson’s disease patients and the normal controls who participated in our research.
Funding
This study was supported by the Science Technology Department of Zhejiang Province (Grant No. 2018C03G1121039), the Fundamental Research Funds for the Central Universities (Grant No. 2018FZA118) and the 13th Five-year Plan for National Key Research and Development Program of China (Grant No. 2016YFC1306600).
Author information
Authors and Affiliations
Contributions
JG designed the study, performed neurological evaluations, statistical analyses, and wrote the first draft. XG performed rsfMRI image preprocessing, statistical analyses and the review and critique of the manuscript. ZC, YC, XD and YL conducted VFSS data acquisition, neurological evaluations and the review and critique of the manuscript. SW, BW and ZO participated in subjects’ collection, VFSS data acquisition and neurological evaluations. MX, QG, XX, PH and MZ helped rsfMRI preprocessing. WL conceived of and organized the research project, participated in the neurological evaluations, and the review and critique of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, J., Guan, X., Cen, Z. et al. Alteration of Brain Functional Connectivity in Parkinson’s Disease Patients with Dysphagia. Dysphagia 34, 600–607 (2019). https://doi.org/10.1007/s00455-019-10015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-019-10015-y