Abstract
High-resolution manometry has traditionally been utilized in gastroenterology diagnostic clinical and research applications. Recently, it is also finding new and important applications in speech pathology and laryngology practices. A High-Resolution Pharyngeal Manometry International Working Group was formed as a grass roots effort to establish a consensus on methodology, protocol, and outcome metrics for high-resolution pharyngeal manometry (HRPM) with consideration of impedance as an adjunct modality. The Working Group undertook three tasks (1) survey what experts were currently doing in their clinical and/or research practice; (2) perform a review of the literature underpinning the value of particular HRPM metrics for understanding swallowing physiology and pathophysiology; and (3) establish a core outcomes set of HRPM metrics via a Delphi consensus process. Expert survey results were used to create a recommended HRPM protocol addressing system configuration, catheter insertion, and bolus administration. Ninety two articles were included in the final literature review resulting in categorization of 22 HRPM-impedance metrics into three classes: pharyngeal lumen occlusive pressures, hypopharyngeal intrabolus pressures, and upper esophageal sphincter (UES) function. A stable Delphi consensus was achieved for 8 HRPM-Impedance metrics: pharyngeal contractile integral (CI), velopharyngeal CI, hypopharyngeal CI, hypopharyngeal pressure at nadir impedance, UES integrated relaxation pressure, relaxation time, and maximum admittance. While some important unanswered questions remain, our work represents the first step in standardization of high-resolution pharyngeal manometry acquisition, measurement, and reporting. This could potentially inform future proposals for an HRPM-based classification system specifically for pharyngeal swallowing disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2017, an independent High-Resolution Pharyngeal Manometry (HRPM) International Working Group was formed as a grassroots cooperative effort between clinicians and research investigators. The primary goal of this group was to facilitate communication between researchers and clinicians with clinical experience utilizing pharyngeal manometry to aid in the translational adoption of this novel technology. The entire Working Group includes 35 members from 20 different institutions (full list of the working group members is included in “Acknowledgments”). Our Mission is to create a standardization of high-resolution pharyngeal manometry acquisition, measurement, reporting, education, and training as well as advocate with payers and healthcare systems. Our Vision is to improve the quality of dysphagia care through the clinical implementation of high-resolution pharyngeal manometry. This Working Group is independent and not sanctioned by any one organization or industry party.
A framework for clinical high-resolution esophageal manometry, called the Chicago Classification, was established through an international consensus process and describes disorders of the esophagogastric junction and disorders of esophageal motility [1]. At the present time, there is no comparable framework for pharyngeal disorders.
The aim of this current initiative was to establish a consensus around protocols and metrics and a diagnostic framework for HRPM. To achieve this, the Working Group undertook three tasks:
- (i)
To recommend a HRPM protocol based on a survey of current expert practices.
- (ii)
To perform a review of the literature to identify evidence underpinning particular HRPM metrics including HRPM with impedance.
- (iii)
To establish a core outcomes set of HRPM metrics via a Delphi consensus process.
Methods
Working Group Survey
A questionnaire was devised to survey the current HRPM practice of experts working in major referral centers who had performed > 200 solid-state HPRM investigations. The respondents (4 United States, 2 Australia, 1 Europe, and 1 New Zealand) identified themselves as speech and language pathologists (71%), physicians/surgeons (29%), and/or scientists (43%). All were engaged in clinical research and most (86%) were in clinical practice where HRPM was being used to aid diagnosis and to guide treatments.
Literature Review
A literature review was undertaken to examine the evidence underpinning pharyngeal pressure topography with and without impedance for the assessment of pharyngeal swallowing physiology and pathophysiology. An initial PubMed search using the search terms ‘manometry’ and ‘pharynx’ identified 832 publications between 1958 and June 2018. The database was then filtered to only include peer-reviewed publications that described pharyngeal pressure recordings using ‘high-resolution’ methodologies. To accomplish this, all Methodology Subgroup members reviewed the list of publications and had the opportunity to add any literature not included in the initial search results that they considered relevant. Papers were considered relevant if they presented pharyngeal and/or UES pressure data based on HRPM, defined as utilizing an array of at least 10 pressure sensors of 1 cm spacing. All papers that did not meet these criteria were deleted from the database, resulting in a total of 92 potentially relevant publications. The relevant publications were then randomly allocated among the Subgroup members who determined whether studies assessed swallow metrics against twelve previously agreed outcome topic areas (Table 2). Each member was then assigned an outcome topic area and undertook a ‘mini-review’ of the relevant publications (HRPM references in Table 2).
Delphi Consensus and Core Outcomes Set
The Working Group’s consensus recommendation was determined via a Delphi process which is an established method for determining core outcomes sets [2]. The Delphi Consensus Group, comprising 10 of the Working Group members currently from 9 different programs, was asked to consider a total of 22 metrics (with associated definitions) and to indicate their support for inclusion of each metric in the core outcomes set. A voting score within the range of 1–9 was used to determine consensus for inclusion; whereby 1–3 indicated decreasing levels of clear disagreement (1 = absolute disagreement), 4 indicated slight disagreement, 5 was neutral, 6 indicated slight agreement, and 7–9 indicated increasing levels of clear agreement (9 = absolute agreement).
In addition to scoring the level of agreement, the Delphi Group was also asked to provide commentary for/against each metric based on available evidence and their experience. When several different metrics described the same phenomena within the same metric class (e.g., contractile pressure integral vs. mean peak pressure vs. absolute peak pressure), the Group was instructed to apportion support to favor the metric they considered to be the ‘best’ over all others.
A priori it was determined that consensus required that at least 70% of the Working Group agreed with the metric (scoring 7–9) and no more than 20% disagreed with the metric (scoring 1–3). All other score distributions were taken to indicate no consensus.
After completion of each round of voting, the scores for all metrics and any for/against commentary was anonymously tabulated and circulated to the Group for consideration before re-scoring. Thus, members could change their score in light of the group’s collective opinion. Voting rounds continued until a stable consensus was reached.
Results
Working Group Survey
The main survey findings are summarized below:
Most respondents (72%) were acquiring both pharyngeal and esophageal data. All had the equipment and catheters to record impedance data and all were equipped to simultaneously acquire radiographic imaging.
Catheter intubation was routinely performed by speech and language pathologists (57%); however, physicians, surgeons, scientists, and nurses were also placing catheters. Most respondents (86%) utilized topical anesthesia to the nasal passages. The Working Group noted a recent trial showing a small reduction in pharyngeal pressures after application of 0.4 ml of 2% viscous lidocaine on a cotton tip applicator [3]. Without further confirmatory evidence, the clinical relevance of the reported change in healthy subjects was considered to be unclear. Previous studies utilizing flexible endoscopic evaluation of swallowing (FEES) [4, 5] have reported increased risk of penetration or aspiration in normal adults with doses of 0.5–1 ml of atomized 4% lidocaine. However, no difference has been observed with smaller amounts (0.2 ml) of atomized lidocaine [6]. Johnson et al. [7] also found no difference in laryngeal sensory testing following nasal anesthesia with 4% cocaine. Multiple studies have shown that topical nasal anesthesia may improve patient comfort and tolerance [4,5,6]. There are no studies evaluating other methods for nasal anesthesia that may avoid pharyngeal spillage, such as nasal packing. Until further evidence is available, the Working Group felt that clinicians should use anesthesia judiciously; by recognizing that use of anesthesia is not always necessary, utilizing the smallest effective dosing and by taking reasonable steps to minimize the spread of anesthetic to the pharyngeal mucosa.
All respondents were applying lubricant gel to aid catheter passage. Having the patient sip water (or a safer consistency if needed) through a straw once the catheter tip enters the pharynx helps with ease of catheter placement. All respondents were performing investigations with the patient sitting or semi-reclined with head in neutral position. Most (57%) were allowing 5 min for accommodation to the catheter prior to administration of test boluses, others were allowing up to 10 min.
Most respondents (71%) used their own standardized test protocol. All reported tested using thin liquid bolus consistencies equivalent to International Dysphagia Diet Standardization Initiative (IDDSI) ‘Level 0’ or SI unit of millipascal second. Other consistencies were variably used; however, extremely thick liquid consistency boluses that may be equivalent to IDDSI ‘Level 4’ were the second most commonly used. It is important to note that respondents were not routinely using flow rate and/or viscosity testing to verify consistency.
All respondents were testing verbally cued bolus swallows. All were administering liquid boluses via syringe while some also reported using spoon, cup, and/or straw. Most (71%) reported that they sometimes incorporated swallow maneuvers and alternative head positions during the procedure (exact maneuvers and positions not specified). Merit of assessing volitional bolus swallowing was not directly surveyed. None of the respondents self-reported assessing natural swallows.
Self-described thin liquid boluses of 5 ml volume were the most commonly used (86%). A few respondents were using < 5 ml volumes (14%) and others were using 10 ml volumes (57%). Most would use larger 15–20 ml volumes. Obtaining three or more repeat swallows was considered sufficient to provide internal consistency of measurements. Overall, the Working Group felt that decisions related to maximum bolus volume and number of repeats tested need to be applied on a ‘case-by-case’ basis influenced by a number of factors including subject tolerance and clinical signs at time of procedure and previously known aspiration and residue risk. Simultaneous videofluoroscopy, while not always necessary, provides for the safest investigation of the highest risk patients.
Recommended HRPM Protocol
Survey responses served as the basis for protocol development (Table 1). This suggested protocol captures the commonalities among the different practices that are indicative of what most of the experts were doing in their clinical/research practice. Consistent themes were (i) the judicious use of topical anesthesia (86%), (ii) a minimum 5-min accommodation period (57%), (iii) bolus delivery via syringe (100%), and (iv) the need to modify consistency of test boluses (71%) that are based on a recipe that ensures the reproducibility of consistencies, either according to the IDDSI framework (http://iddsi.org/) or the use of SI Units of viscosity (mPa s) as has been recommended by The European Society for Swallowing Disorders [8].
Literature Review
A range of specific HRPM phenomena were found to be considered diagnostically important [9, 10] (Table 2). As a starting point, the Working Group focused its efforts on exploring swallow metric classes that describe four swallow phenomena. These features were (i) lumen occlusive pressures generated at the velum, mesopharynx, hypopharynx, and UES, (ii) hypopharyngeal intrabolus pressure, (iii) UES relaxation pressures, and (iv) UES opening duration and extent. The major findings are summarized below and in Table 3, which illustrates how these swallow phenomena have been shown to relate to outcomes, providing an indication as to their relative importance as diagnostic measures.
(i) Pharyngeal and UES Lumen Occlusive Pressures
Lumen occlusive pressures generated by muscle contraction within pharyngeal and UES regions are commonly measured to identify specific regional impairments that may guide therapeutic strategies to improve contractile function; although this remains to be formally tested. Metrics described defined pressures within four anatomical regions (velo-, meso-, hypopharynx, and UES; Fig. 1a–c) that were assessed independently or combined (e.g., velo + mesopharynx [11,12,13], meso + hypopharynx [14], or the whole pharynx [15, 16]). UES occlusive pressures were measured pre-deglutitive or post-deglutitive (Fig. 1b). Pressures generated by the velopharyngeal region are typically sustained and multimodal [17] (Fig. 1b) and mechanistically serve to seal the nasopharynx when the pharynx is being challenged with large boluses. Velopharyngeal pressure increases with bolus volume [11, 12, 14] and with inverted body positioning [18]. In contrast, the regions inferior to the velopharynx appear to be less consistently affected with some reports of higher pressure with increased bolus size [19, 20] and bolus consistency [12]. Pressures throughout the pharynx and UES have been measured to be weaker in patients with dysphagia symptoms or in relation the dysphagia sequelae such as aspiration [16, 20,21,22,23,24,25]. Global and regional weakness of the pharynx is undoubtedly a feature of some specific pathologies [12, 20, 23]. However, studies that include a large sample (75 + patients) only describe data averaged across broad etiologies [24, 26, 27].
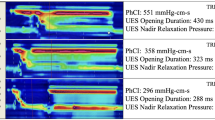
HRPM with simultaneous videofluoroscopy. a An example pharyngeal pressure topography plot during cued volitional swallowing of a 10 ml thin liquid barium bolus (IDDSI 0). Vertical lines correspond to the time points of the two radiographic images in C. b Individual pressure signals recorded by sensors at the different axial locations along the pharynx. The four graphs show the individual pressure signals occurring within each anatomical region and illustrate the variable nature of pressures recorded throughout the pharynx. Vertical lines correspond to the time points of the two radiographic images in (c). c Radiographic images before and during the swallow. The catheter in situ is visible in the image and the relevant pressure sensor numbers are labeled. Technical Details: The measurements were performed in a 42-year-old male subject using a 2.75-mm-diameter solid-state high-resolution manometry catheter incorporating 36 1-cm-spaced pressure sensors (Given Imaging). Data were acquired at 50 samples/s (Manoscan, Given Imaging, USA) and analyzed using MATLAB (The MathWorks Inc., Natick, MA, USA)
Measurement of the contractile peak pressure has the practical advantage of being easy to determine without highly specialized software. The concept of a contractile integral, which defines pressure over space and time (mean pressure × duration × length), has gained in popularity as a measure of the ‘vigor’ of the pharyngeal swallowing response [15, 16]. Altered pharyngeal pressure in relation to aging, during swallow exercises, and with volume challenges have been detected by both peak pressures and pressure integrals [12, 14, 15, 28].
The added value of a pharyngeal contractile integral, over peak pressure is not proven at this time. However, it can be argued that a contractile integral may have greater value for recording pressures within the velopharyngeal and mesopharyngeal regions in particular, because pressure generation within these regions displays typically sustained and/or multimodal features, in contrast to the characteristically brief and single peaked pressures generated by the hypopharynx (Fig. 1b).
(ii) Hypopharyngeal Intrabolus Pressure
Hypopharyngeal intrabolus pressure (IBP) is a marker of UES restriction to bolus flow during swallowing. Abnormal IBP suggests an increased pressure gradient across the pharyngo-esophageal junction that may drive reciprocal compensatory changes in upstream functions, such as augmented meso- or velopharyngeal pressure generation. Bolus flow restriction can be rectified following cricopharyngeal myotomy or dilatation [29,30,31] but a recent systematic review revealed inadequate evidence to guide clinical decision making [32]. The HRPM literature shows that hypopharyngeal IBP physiologically increases with bolus size and consistency [11, 12, 14, 19, 33], is elevated in some patient cohorts [12, 13, 21, 22], elevated in relation to dysphagia sequelae [19, 21, 27, 34], and following exposure to opioids [35, 36]. There are methodological challenges associated with reliably defining IBP. Three approaches are below:
- (i)
Mid-hypopharyngeal IBP, defined by the pressure within the advancing bolus measured at the midpoint of radiologically determined bolus flow [17, 33].
- (ii)
Hypopharyngeal pressure increment (HPI), defined by the average pressures preceding the onset of the upstroke of pharyngeal contraction to a 20 mmHg threshold [14] (Fig. 2b).
Fig. 2 HRPM measures of intrabolus pressure, UES relaxation and UES opening. a An example pharyngeal pressure topography plot of the entire pharyngo-esophageal segment during cued volitional swallowing of a 20 ml thin liquid bolus (IDDSI 0). b Individual pressure (black lines) and admittance (pink) signals recorded at different axial locations along the pharyngo-esophageal segment. Note: Admittance is the inverse product of impedance (1/impedance; units in millisiemens, mS) and therefore rises in relation to bolus presence. The time of maximum admittance (≡ nadir impedance) is identified by ‘x’; this marks maximum bolus distension at each location along the pharynx allowing the trajectory of bolus movement to be mapped over time and space during the swallow (also illustrated by the pink line in Panel A). Knowing when maximum bolus distension occurs allows the corresponding intrabolus distension pressure to be determined; known as the pressure at nadir impedance (PNI). The discrete PNI that is measured at 1 cm proximal of the UES apogee position is a validated marker of pharyngeal flow resistance (orange squares in Panels a and b). Another marker is the hypopharyngeal pressure increment (HPI) which is determined by averaging all hypopharyngeal pressures preceding the onset of the upstroke of pharyngeal contraction to a 20 mmHg threshold; shown in this example as the average of pressures between points ‘a’ and ‘b’. c The time-profile of upper esophageal sphincter (UES) pressure (black line) based on the ‘e-sleeve’ method (utilizing ‘max P.’ in Panel A) and the corresponding UES admittance (pink). The UES nadir pressure and UES integrated relaxation pressure (UES IRP) measure the extent of pressure relaxation. UES IRP is the median of the lowest 0.25 s of UES pressure (non-contiguous; see red squares during relaxation). UES maximum admittance (‘x’) measures the extent of UES opening. d Average data showing increasing intrabolus pressure (flow resistance) and UES admittance (UES opening) when the subject was challenged with larger bolus volumes. Technical Details: The measurements were performed in a 55-year-old female subject using a 2.7-mm-diameter solid-state high-resolution impedance-manometry catheter with 32 1-cm-spaced pressure sensors and 16 (2 cm) impedance segments (Unisensor AG, Attikon, Switzerland). Data were acquired at 20 samples/s (Solar GI HRM system, MMS Enschede, The Netherlands) and analyzed via the Swallow Gateway open access analysis portal (https://www.swallowgateway.com)
- (iii)
Hypopharyngeal pressure at nadir impedance (PNadImp or PNI), defined by the pressure at the time of maximum hypopharyngeal distension deduced by impedance topography [21, 37] (Fig. 2b). IBP defined in this way has been considered the impedance-based equivalent of mid-IBP [38].
All methods for calculating IBP have their limitations. Mid-IBP has a long history of use prior to the advent of HRPM [29]; however, measurement requires simultaneous videofluoroscopy and perfectly synchronized image analysis that can be time consuming. The average pressure increment has only been reported in one study of non-dysphagic patients [14] and has not been assessed in relation to pathologic swallowing. PNI requires impedance recording which does not always come as standard adding to the device cost.
Mid-IBP and PNI were found in our review to be the most utilized measures of IBP. PNI is the only IBP metric tested for intra- and inter-rater reliability [13, 34, 39, 40]. Original reports calculated the average PNI for the entire hypopharyngeal region proximal to the UES [21, 34, 41]; however, this has been recently refined to only utilize the discrete nadir impedance pressure measured 1 cm proximal to the UES apogee position [12, 13, 35, 37, 40]. This iteration of the metric (Fig. 2a, b) has been validated as a measure of augmented pharyngeal flow resistance [35] and, among a range of different measures, appears to be the most predictive of strictures following head and neck cancer treatments [37].
A final cautionary point for any hypopharyngeal HRPM measures is that they may be subject to hypopharyngeal pressure transients predominantly caused by contact of the tilting epiglottis with pressure sensors along the catheter [42]. Any analysis should ideally recognize such pressures because i) they are potentially erroneous and therefore can influence numerical values generated for IBP and ii) they may reliably predict epiglottic inversion that may have clinical relevance for some patients.
(iii) UES Relaxation Pressure
UES pressures have been shown to be a direct correlate of cricopharyngeal electromyography and therefore are indicative of brain stem mediated activation and deactivation of cricopharyngeus muscle [43,44,45,46]. UES pressures during bolus swallowing and for the period of greatest relaxation are also indirect measures of IBP, physiologically increasing with bolus size [11, 14, 46] and elevated in some patient cohorts compared to controls [12, 20, 22, 24, 44] as well as in relation to dysphagia sequelae [13, 47, 48]. UES relaxation pressures also increase with age [15, 22, 36], inverted body positioning [18], and following exposure to opioids [35, 36].
The advent of HRPM has greatly improved assessment of UES relaxation pressures by utilizing the e-sleeve method [49, 50] to accommodate the sometimes significant elevation of the UES during swallowing [51]. This method measures all axial pressures within the limits of UES high-pressure zone and ‘maps’ the dynamic movement of the UES high-pressure zone over time based on the location of maximum axial pressure (Fig. 2a, c). UES relaxation parameters can then be derived from the profile of maximal pressures over time [22, 49, 52]. Two dominant approaches to defining UES relaxation pressure are described in the HRPM literature, namely the minimum relaxation pressure (or nadir pressure) and the integrated relaxation pressure (Fig. 2c). UES integrated relaxation pressure (UES IRP) quantifies the lowest non-consecutive 0.20–0.25 s of UES pressure during relaxation (Fig. 2c). UES IRP emulates the esophagogastric junction ‘IRP 4s’ that is widely used for the diagnosis of achalasia and esophageal outflow obstruction [1]. In esophageal diagnosis, IRP has been shown to be superior to discrete nadir relaxation pressure for distinguishing abnormal EGJ relaxation [50]. There is a paucity of directly comparative data from HRPM studies evaluating both UES nadir pressure and UES IRP. However, as UES high-pressure zone and catheter can move independently over the active swallow period, it could be argued that UES IRP, being based on a series of measurements, may be more comprehensive than taking a single data point of lowest pressure.
(iv) UES Opening Impedance
The mechanisms that determine UES opening extent include strength and timing of supra- and infra-hyoid muscle activation and the distension pressure generated by the swallowed bolus driven by pharyngeal propulsion. Additionally, UES opening is dependent upon the appropriate reciprocal neural deactivation and compliance of the cricopharyngeus and associated muscles and structures [10, 42,43,44,45,46].
Bolus impedance is a direct correlate of luminal diameter/area and therefore intraluminal impedance (or the inverse product called ‘admittance,’ Fig. 2c) has been used to measure the timing and extent of opening of the UES [44, 53]. UES impedance during bolus swallows, correlates with bolus size [11, 12, 46] (Fig. 2d) and is altered in patient cohorts compared to controls [12, 13, 22, 44, 53, 54] and with aging [44, 55].
Delphi Consensus and Core Outcomes Set
A total of four voting rounds were required to reach a stable level of agreement on all 22 evaluated metrics of which consensus to recommend was achieved for eight metrics. The recommended swallow metrics and key comments expressed during the Delphi rounds are detailed in Tables 4 and 5. The recommended metrics allow characterization of diagnostically important swallowing phenomena.
Discussion
The major outcomes of this work were the development of a recommendation for a standard HRPM protocol and an associated core outcomes set of recommended diagnostic measures. This work can potentially inform future proposals for a HRPM-based classification system specifically for characterization of pharyngeal swallowing disorders.
As part of the process the determining a consensus-based framework, the Working Group undertook an extensive literature review that provided the evidence underpinning what clinicians performing HRPM should seek to measure. The knowledge bank of HRPM literature is growing but, for many reasons, the literature is incomplete and complex to decipher. Nevertheless, the review suggests that published individual swallow metrics can be meaningfully consolidated (Table 3).
Specification of a recommended core outcomes set of pressure and/or impedance-derived swallow metrics provides a starting point for the development of a framework for scientific and clinical communication. The Working Group’s consensus recommendation was determined via the Delphi process, which is a widely used and accepted method for gathering data from a panel of experts involving a formal group communications and several rounds of discussion and voting.
The current work has multiple limitations that are important to discuss. The Working Group did not address technological questions regarding factors that can influence the quality and reliability of measurements such as data and image synchronization and sampling frequency, catheter diameter and pressure sensor type, spacing and orientation. These are important issues to be investigated in the future. The varied use of different manufactured catheters and software may preclude some clinicians from being able to utilize impedance metrics. They are included, however, in the recommended outcomes set as these impedance measures had significant evidence in the literature to suggest clinical utility and gained majority support in the Delphi consensus process. While methods for calculation of the different metrics were provided, we recognize that multiple approaches can be found in the literature and we do not suggest that one method of calculation is superior to another.
Despite growing enthusiasm for HRPM as a diagnostic modality, how HRPM can influence patient management remains unclear. The ability of HRPM to predict outcomes, determine therapeutic effects, monitor disease recovery or progression or enable biofeedback training is currently unknown. Of particular interest may be the effect of pharyngeal swallowing maneuvers on HRPM measurements and their role in evaluating efficacy or guiding clinical practice. There are also still unanswered questions regarding protocol standardization, particularly in relation to recommended bolus consistency administration. At this time, there is no current consensus or evidence to suggest the appropriate bolus consistencies in either diagnostic or therapeutic examinations.
Furthermore, the current work does not address critically important questions around where the limits of HRPM lie, as a standalone test. These include: When is an HRPM-only procedure appropriate? What are the circumstances under which adjunct radiology should be considered essential? Should HRPM be preserved as a comprehensive, stand alone, pharyngeal examination to diagnose a pharyngeal dysfunction only or should a standardized protocol allow for (limited) assessments below the esophageal transition zone? If esophageal dysfunction is present, which in some populations may be frequently (as recently reported for laryngectomy patients [56]), then what esophageal findings should be considered relevant to symptoms? These and other issues are challenging to address and will raise many questions and concerns among experts in the field.
Conclusion
A recommended protocol and outcomes set of diagnostic measures was determined following an extensive evidence-based process. This work represents the first step in an evolving process to establish both clinical and research HRPM guidelines and will require revision based on future objective evidence. It is envisaged that a classification framework for pharyngeal disorders, akin to Chicago Classification, could ultimately emerge from this and future efforts.
References
International High Resolution Manometry Working, G. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74. https://doi.org/10.1111/nmo.12477.
Williamson PR, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132.
Guiu Hernandez E, Gozdzikowska K, Apperley O, Huckabee ML. Effect of topical nasal anesthetic on swallowing in healthy adults: a double-blind, high-resolution manometry study. Laryngoscope. 2017;128(6):1335–9. https://doi.org/10.1002/lary.26996.
Fife TA, et al. Use of topical nasal anesthesia during flexible endoscopic evaluation of swallowing in dysphagic patients. Ann Otol Rhinol Laryngol. 2015;124:206–11.
Lester S, et al. The effects of topical anesthetic on swallowing during nasoendoscopy. Laryngoscope. 2013;123:1704–8.
O’Dea MB, et al. Effect of lidocaine on swallowing during FEES in patients with dysphagia. Ann Otol Rhinol Laryngol. 2015;124:537–44.
Johnson PE, Belafsky PC, Postma GN. Topical nasal anesthesia and laryngopharyngeal sensory testing: a prospective, double-blind crossover study. Ann Otol Rhinol Laryngol. 2003;112:14–6.
Newman R, Vilardell N, Clavé P, Speyer R. Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: white paper by the European Society for Swallowing Disorders (ESSD). Berlin: Springer; 2016.
Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia. 2014;29:2–16. https://doi.org/10.1007/s00455-013-9494-5.
Cock C, Omari T. Diagnosis of swallowing disorders: how we interpret pharyngeal manometry. Curr Gastroenterol Rep. 2017;19:11. https://doi.org/10.1007/s11894-017-0552-2.
Ferris L, et al. Characterization of swallow modulation in response to bolus volume in healthy subjects accounting for catheter diameter. Laryngoscope. 2017;28(6):1328–34. https://doi.org/10.1002/lary.26820.
Schar M, et al. Pathophysiology of swallowing following oropharyngeal surgery for obstructive sleep apnea syndrome. Neurogastroenterol Motil. 2017;30(5):e13277. https://doi.org/10.1111/nmo.13277.
Singendonk M, et al. Reliability of an online analysis platform for pharyngeal high-resolution impedance manometry (HRIM) recordings. Speech Lang Hear. 2018;154:S983. https://doi.org/10.1016/S0016-5085(18)33298-0.
Jiao H, et al. A human model of restricted upper esophageal sphincter opening and its pharyngeal and UES deglutitive pressure phenomena. Am J Physiol. 2016;311:G84–90. https://doi.org/10.1152/ajpgi.00145.2016.
Nativ-Zeltzer N, Logemann JA, Zecker SG, Kahrilas PJ. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography: a normative study of younger and older adults. Neurogastroenterol Motil. 2016;28:721–31. https://doi.org/10.1111/nmo.12769.
O’Rourke A, Humphries K, Lazar A, Martin-Harris B. The pharyngeal contractile integral is a useful indicator of pharyngeal swallowing impairment. Neurogastroenterol Motil. 2017;29:e131444. https://doi.org/10.1111/nmo.13144.
Walczak CC, Jones CA, McCulloch TM. Pharyngeal pressure and timing during bolus transit. Dysphagia. 2017;32:104–14. https://doi.org/10.1007/s00455-016-9743-5.
Rosen SP, Abdelhalim SM, Jones CA, McCulloch TM. Effect of body position on pharyngeal swallowing pressures using high-resolution manometry. Dysphagia. 2017;32:835–6. https://doi.org/10.1007/s00455-017-9866-3.
Omari TI, Dejaeger E, Tack J, Van Beckevoort D, Rommel N. Effect of bolus volume and viscosity on pharyngeal automated impedance manometry variables derived for broad dysphagia patients. Dysphagia. 2013;28:146–52. https://doi.org/10.1007/s00455-012-9423-z.
Lippert D, et al. Preliminary evaluation of functional swallow after total laryngectomy using high-resolution manometry. Ann Otol Rhinol Laryngol. 2016;125:541–9. https://doi.org/10.1177/0003489416629978.
Omari TI, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140:1454–63. https://doi.org/10.1053/j.gastro.2011.02.051.
Cock C, et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterol Motil. 2016;28:225–33. https://doi.org/10.1111/nmo.12714.
Jones CA, Ciucci MR. Multimodal swallowing evaluation with high-resolution manometry reveals subtle swallowing changes in early and mid-stage Parkinson disease. J Parkinson’s Dis. 2016;6:197–208. https://doi.org/10.3233/JPD-150687.
Park C-H, et al. Quantitative analysis of swallowing function between dysphagia patients and healthy subjects using high-resolution manometry. Ann Rehabil Med. 2017;41:776–85.
Park D, Oh Y, Ryu JS. Findings of abnormal videofluoroscopic swallowing study identified by high-resolution manometry parameters. Arch Phys Med Rehabil. 2016;97:421–8. https://doi.org/10.1016/j.apmr.2015.10.084.
Park C-H, et al. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am J Gastroenterol. 2017;112:1074. https://doi.org/10.1038/ajg.2017.81.
Kritas S, Dejaeger E, Tack J, Omari T, Rommel N. Objective prediction of pharyngeal swallow dysfunction in dysphagia through artificial neural network modeling. Neurogastroenterol Motil. 2016;28:336–44. https://doi.org/10.1111/nmo.12730.
Doeltgen SH, Ong E, Scholten I, Cock C, Omari T. Biomechanical quantification of mendelsohn maneuver and effortful swallowing on pharyngoesophageal function. Otolaryngol Head Neck Surg. 2017;157:816–23. https://doi.org/10.1177/0194599817708173.
Cook IJ, et al. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology. 1992;103:1229–35.
Ali GN, Wallace KL, Laundl TM, Hunt DR, Cook IJ. Predictors of outcome following cricopharyngeal disruption for pharyngeal dysphagia. Dysphagia. 1997;12:133–9.
Mason RJ, et al. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg. 1998;228:598.
Knigge MA, Thibeault SL. Swallowing outcomes after cricopharyngeal myotomy: a systematic review. Head Neck. 2018;40:203–12.
Zhang T, et al. Biomechanics of pharyngeal deglutitive function following total laryngectomy. Otolaryngol Head Neck Surg. 2016;155:295–302. https://doi.org/10.1177/0194599816639249.
Omari TI, et al. Reproducibility and agreement of pharyngeal automated impedance manometry with videofluoroscopy. Clin Gastroenterol Hepatol. 2011;9:862–7. https://doi.org/10.1016/j.cgh.2011.05.026.
Doeltgen SH, Omari TI, Savilampi J. Remifentanil alters sensory neuromodulation of swallowing in healthy volunteers: quantification by a novel pressure impedance analysis. Am J Physiol. 2016;310:G1176–82. https://doi.org/10.1152/ajpgi.00138.2016.
Savilampi J, Omari T, Magnuson A, Ahlstrand R. Effects of remifentanil on pharyngeal swallowing: a double blind randomised cross-over study in healthy volunteers. Eur J Anaesthesiol. 2016;33:622–30. https://doi.org/10.1097/EJA.0000000000000461.
Szczesniak M, Wu P, Maclean J, Omari T, Cook I. The critical importance of pharyngeal contractile forces on the validity of intrabolus pressure as a predictor of impaired pharyngo-esophageal junction compliance. Neurogastroenterol Motil. 2018;30:e13374. https://doi.org/10.1111/nmo.13374.
Cook IJ. Combined pharyngeal impedance-manometry: has it finally come of age? Clin Gastroenterol Hepatol. 2011;9:813–5.
Szczesniak M, et al. Inter-rater reliability and validity of automated impedance manometry analysis and fluoroscopy in dysphagic patients after head and neck cancer radiotherapy. Neurogastroenterol Motil. 2015;27:1183–9. https://doi.org/10.1111/nmo.12610.
Omari TI, et al. The reliability of pharyngeal high resolution manometry with impedance for derivation of measures of swallowing function in healthy volunteers. Int J Otolaryngol. 2016;2016:1. https://doi.org/10.1155/2016/2718482.
Omari TI, et al. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol. 2011;106:1796–802. https://doi.org/10.1038/ajg.2011.143.
Park D, et al. Normal contractile algorithm of swallowing related muscles revealed by needle EMG and its comparison to videofluoroscopic swallowing study and high resolution manometry studies: a preliminary study. J Electromyogr Kinesiol. 2017;36:81–9. https://doi.org/10.1016/j.jelekin.2017.07.007.
Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Ann Otol Rhinol Laryngol. 2014;123:174–82. https://doi.org/10.1177/0003489414522975.
Omari TI, et al. Upper esophageal sphincter mechanical states analysis: a novel methodology to describe UES relaxation and opening. Front Syst Neurosci. 2015;8:241. https://doi.org/10.3389/fnsys.2014.00241.
Omari TI, et al. Predicting the activation states of the muscles governing upper esophageal sphincter relaxation and opening. Am J Physiol. 2016;310:G359–66. https://doi.org/10.1152/ajpgi.00388.2015.
Cock C, Jones CA, Hammer MJ, Omari TI, McCulloch TM. Modulation of upper esophageal sphincter (UES) relaxation and opening during volume swallowing. Dysphagia. 2017;32:216–24. https://doi.org/10.1007/s00455-016-9744-4.
Lee T, et al. Failed deglutitive upper esophageal sphincter relaxation is a risk factor for aspiration in stroke patients with oropharyngeal dysphagia. J Neurogastroenterol Motil. 2017;23:34–40. https://doi.org/10.5056/jnm16028.
Rommel N, et al. Objective assessment of swallow function in children with suspected aspiration using pharyngeal automated impedance manometry. J Pediatr Gastroenterol Nutr. 2014;58:789–94. https://doi.org/10.1097/MPG.0000000000000337.
Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol. 2006;291:G525–31. https://doi.org/10.1152/ajpgi.00081.2006.
Ghosh SK, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol. 2007;293:G878–85.
Kahrilas P, Dodds W, Dent J, Logemann J, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62.
Weijenborg PW, Kessing BF, Smout AJ, Bredenoord AJ. Normal values for solid-state esophageal high-resolution manometry in a European population; an overview of all current metrics. Neurogastroenterol Motil. 2014;26:654–9. https://doi.org/10.1111/nmo.12314.
Omari TI, et al. Upper esophageal sphincter impedance as a marker of sphincter opening diameter. Am J Physiol. 2012;302:G909–13. https://doi.org/10.1152/ajpgi.00473.2011.
Ferris L, et al. Pressure-flow analysis for the assessment of pediatric oropharyngeal dysphagia. J Pediatr. 2016;177:279–285-e271. https://doi.org/10.1016/j.jpeds.2016.06.032.
Omari T, et al. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol Motil. 2014;26:59–68. https://doi.org/10.1111/nmo.12224.
Zhang T, et al. Esophageal dysmotility in patients following total laryngectomy. Otolaryngol Head Neck Surg. 2017;158(2):323–30. https://doi.org/10.1177/0194599817736507.
McCulloch TM, Hoffman MR, Ciucci MR. High resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119:369–76.
Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. Laryngoscope. 2010;120:2367–73. https://doi.org/10.1002/lary.21150.
Omari T, Dejaeger E, Tack J, Vanbeckevoort D, Rommel N. An impedance-manometry based method for non-radiological detection of pharyngeal postswallow residue. Neurogastroenterol Motil. 2012;24:e277–84. https://doi.org/10.1111/j.1365-2982.2012.01931.x.
Omari T, Kritas S, Cock C. New insights into pharyngo-esophageal bolus transport revealed by pressure-impedance measurement. Neurogastroenterol Motil. 2012;24:e549–56. https://doi.org/10.1111/nmo.12007.
Silva LC, et al. Anatomophysiology of the pharyngo-upper esophageal area in light of high-resolution manometry. J Gastrointest Surg. 2013;17:2033–8. https://doi.org/10.1007/s11605-013-2358-3.
Hammer MJ, Jones CA, Mielens JD, Kim CH, McCulloch TM. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia. 2014;29:564–70. https://doi.org/10.1007/s00455-014-9545-6.
Kim CK, et al. Effects of head rotation and head tilt on pharyngeal pressure events using high resolution manometry. Ann Rehabil Med. 2015;39:425–31. https://doi.org/10.5535/arm.2015.39.3.425.
Rosen SP, Jones CA, McCulloch TM. Pharyngeal swallowing pressures in the base-of-tongue and hypopharynx regions identified with three-dimensional manometry. Laryngoscope. 2017;127:1989–95. https://doi.org/10.1002/lary.26483.
Balasubramanian G, et al. Characterization of pharyngeal peristaltic pressure variability during volitional swallowing in healthy individuals. Neurogastroenterol Motil. 2017;29:e13119. https://doi.org/10.1111/nmo.13119.
Lin T, et al. Effect of bolus volume on pharyngeal swallowing assessed by high-resolution manometry. Physiol Behav. 2014;128:46–51. https://doi.org/10.1016/j.physbeh.2014.01.030.
Omari TI, et al. Assessment of intraluminal impedance for the detection of pharyngeal bolus flow during swallowing in healthy adults. Am J Physiol. 2006;290:G183–8. https://doi.org/10.1152/ajpgi.00011.2005.
Takasaki K, et al. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729–32. https://doi.org/10.1097/MLG.0b013e31817dfd02.
Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res. 2012;55:892–902. https://doi.org/10.1044/1092-4388(2011/11-0088).
Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-resolution manometry data. Laryngoscope. 2013;123:1746–53. https://doi.org/10.1002/lary.23987.
Ferris L, et al. Pressure flow analysis in the assessment of preswallow pharyngeal bolus presence in dysphagia. Int J Otolaryngol. 2015;2015:1–6. https://doi.org/10.1155/2015/764709.
Noll L, Rommel N, Davidson G, Omari T. Pharyngeal flow interval: a novel impedance-based parameter correlating with aspiration. Neurogastroenterol Motil. 2011;23:551. https://doi.org/10.1111/j.1365-2982.2010.01634.x.
Hoffman MR, et al. Artificial neural network classification of pharyngeal high-resolution manometry with impedance data. Laryngoscope. 2013;123:713–20. https://doi.org/10.1002/lary.23655.
Hoffman MR, et al. Classification of high-resolution manometry data according to videofluoroscopic parameters using pattern recognition. Otolaryngol Head Neck Surg. 2013;149:126–33. https://doi.org/10.1177/0194599813489506.
Yoon KJ, Park JH, Park JH, Jung IS. Videofluoroscopic and manometric evaluation of pharyngeal and upper esophageal sphincter function during swallowing. J Neurogastroenterol Motil. 2014;20:352–61. https://doi.org/10.5056/jnm14021.
Lan Y, et al. Biomechanical changes in the pharynx and upper esophageal sphincter after modified balloon dilatation in brainstem stroke patients with dysphagia. Neurogastroenterol Motil. 2013;25:e821–9. https://doi.org/10.1111/nmo.12209.
Takasaki K, Umeki H, Hara M, Kumagami H, Takahashi H. Influence of effortful swallow on pharyngeal pressure: evaluation using a high-resolution manometry. Otolaryngol Head Neck Surg. 2011;144:16–20. https://doi.org/10.1177/0194599810390885.
Hoffman MR, et al. High resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27:418–26. https://doi.org/10.1007/s00455-011-9385-6.
Derrey S, et al. Impact of deep brain stimulation on pharyngo-esophageal motility: a randomized cross-over study. Neurogastroenterol Motil. 2015;27:1214–22. https://doi.org/10.1111/nmo.12607.
Jungheim M, Schubert C, Miller S, Ptok M. Swallowing function after continuous neuromuscular electrical stimulation of the submandibular region evaluated by high-resolution manometry. Dysphagia. 2017;32:501–8. https://doi.org/10.1007/s00455-017-9791-5.
Doeltgen SH, Rigney L, Cock C, Omari T. Effects of cortical anodal transcranial direct current stimulation on swallowing biomechanics. Neurogastroenterol Motil. 2018;30:13434. https://doi.org/10.1111/nmo.13434.
Jones CA, et al. Reliability of an automated high-resolution manometry analysis program across expert users, novice users, and speech-language pathologists. J Speech Lang Hear Res. 2014;57:831–6. https://doi.org/10.1044/2014_JSLHR-S-13-0101.
Lee TH, et al. High-resolution manometry: reliability of automated analysis of upper esophageal sphincter relaxation parameters. Turk J Gastroenterol. 2014;25:473–80. https://doi.org/10.5152/tjg.2014.8021.
Jungheim M, et al. Calculation of upper esophageal sphincter restitution time from high resolution manometry data using machine learning. Physiol Behav. 2016;165:413–24. https://doi.org/10.1016/j.physbeh.2016.08.005.
Kern MK, et al. Pharyngeal peristaltic pressure variability, operational range, and functional reserve. Am J Physiol. 2017;312:G516–25. https://doi.org/10.1152/ajpgi.00382.2016.
Williams RB, Pal A, Brasseur JG, Cook IJ. Space–time pressure structure of pharyngo-esophageal segment during swallowing. Am J Physiol. 2001;281:G1290–300.
Plowman EK, et al. Autologous myoblasts attenuate atrophy and improve tongue force in a denervated tongue model: a pilot study. Laryngoscope. 2014;124:E20–6. https://doi.org/10.1002/lary.24352.
Jones C, et al. Identification of swallowing disorders in early and mid-stage Parkinson’s disease using pattern recognition of pharyngeal high-resolution manometry data. Neurogastroenterol Motil. 2017;30(4):e13236.
Menezes MA, Herbella FA, Patti MG. High-resolution manometry evaluation of the pharynx and upper esophageal sphincter motility in patients with achalasia. J Gastrointest Surg. 2015;19:1753–7. https://doi.org/10.1007/s11605-015-2901-5.
Arenaz Búa B, Olsson R, Westin U, Rydell R. The pharyngoesophageal segment after total laryngectomy. Ann Otol Rhinol Laryngol. 2017;126:138–45. https://doi.org/10.1177/0003489416681321.
Knigge MA, Thibeault S. Relationship between tongue base region pressures and vallecular clearance. Dysphagia. 2016;31:391–7. https://doi.org/10.1007/s00455-015-9688-0.
Meyer JP, Jones CA, Walczak CC, McCulloch TM. Three-dimensional manometry of the upper esophageal sphincter in swallowing and nonswallowing tasks. Laryngoscope. 2016;126:2539–45. https://doi.org/10.1002/lary.25957.
Hutcheson KA, Hammer MJ, Rosen SP, Jones CA, McCulloch TM. Expiratory muscle strength training evaluated with simultaneous high resolution manometry and electromyography. Laryngoscope. 2017;127:797–804. https://doi.org/10.1002/lary.26397.
de Leon A, Thörn S-E, Wattwil M. High-resolution solid-state manometry of the upper and lower esophageal sphincters during anesthesia induction: a comparison between obese and non-obese patients. Anesth Analg. 2010;111:149–53. https://doi.org/10.1213/ANE.0b013e3181e1a71f.
Mielens JD, Hoffman MR, Ciucci MR, Jiang JJ, McCulloch TM. Automated analysis of pharyngeal pressure data obtained with high-resolution manometry. Dysphagia. 2011;26:3–12. https://doi.org/10.1007/s00455-010-9320-2.
Vardar R, Sweis R, Anggiansah A, Wong T, Fox M. Upper esophageal sphincter and esophageal motility in patients with chronic cough and reflux: assessment by high-resolution manometry. Dis Esophagus. 2013;26:219–25. https://doi.org/10.1111/j.1442-2050.2012.01354.x.
Pinna BR, Herbella FA, de Biase N, Vaiano TC, Patti MG. High-resolution manometry evaluation of pressures at the pharyngo-upper esophageal area in patients with oropharyngeal dysphagia due to vagal paralysis. Dysphagia. 2017;32:657–62. https://doi.org/10.1007/s00455-017-9811-5.
Singendonk M, et al. Upper gastrointestinal function in morbidly obese adolescents before and 6 months after gastric banding. Obes Surg. 2017;28:1277–88. https://doi.org/10.1007/s11695-017-3000-3.
Rommel N, Davidson G, Cain T, Hebbard G, Omari T. Videomanometric evaluation of pharyngo-oesophageal dysmotility in children with velocardiofacial syndrome. J Pediatr Gastroenterol Nutr. 2008;46:87–91.
Ferris L, et al. Piecemeal deglutition and the implications for pressure impedance dysphagia assessment in pediatrics. J Pediatr Gastroenterol Nutr. 2018;67:713–9. https://doi.org/10.1097/MPG.0000000000002080.
Jones CA, et al. Methods for measuring swallowing pressure variability using high-resolution manometry. Front Appl Math Stat. 2018;4:23. https://doi.org/10.3389/fams.2018.00023.
Hernandez EG, Gozdzikowska K, Jones R, Huckabee M-L. Comparison of unidirectional and circumferential manometric measures within the pharyngoesophageal segment: an exploratory study. Eur Arch Otorhinolaryngol. 2018;275:2302–10.
Jadcherla SR, et al. Defining pharyngeal contractile integral during high-resolution manometry in neonates: a neuromotor marker of pharyngeal vigor. Pediatr Res. 2018;84:341–7.
Acknowledgements
Additional High-Resolution Pharyngeal Manometry International Working Group Members: Jacqui Allen, University of Auckland; Lee Askt, Johns Hopkins University; Peter Belafsky, University of California, Davis; Giselle Carnaby, University of Central Florida; Charles Cock, Flinders University; Michael Crary, University of Central Florida; Kate Davidson, Medical University of South Carolina; Sebastian Doeltgen, Flinders University; Kathleen Huber, University of Wisconsin; Maggie-Lee Huckabee, University of Canterbury; Ianessa Humbert, University of Florida; Jan Lewin, MD Anderson Cancer Center; Phoebe Macrae, University of Canterbury; Bonnie Martin-Harris, Northwestern University; Nancy McCulloch, Emory University; Timothy McCulloch, University of Wisconsin; Barbara Messing, Greater Baltimore Medical Center; Anna Miles, University of Auckland; Joseph Murray, Veterans Administration Hospital, Ann Arbor; Jessica Pisegna, Boston Medical Center; Gregory Postma, Medical College of Georgia; Michal Szczesniak, University of New South Wales.
Funding
Medtronic (educational funding), Medical University of South Carolina Department of Otolaryngology – Head and Neck Surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Taher Omari declares that he is a co-inventor of a relevant patent (AU2011301768 Patentee: Women’s and Children’s Health Network Incorporated). Michelle Ciucci declares that she has no conflict of interest. Kristin Gozdzikowska declares that she has no conflict of interest. Esther Hernández declares that she has no conflict of interest. Katherine Hutcheson declares that she has a travel stipend from Medtronic Inc. Corinne Jones declares that she has no conflict of interest. Julia Maclean declares that she has no conflict of interest. Nogah Nativ-Zeltzer declares that she has no conflict of interest. Emily Plowman declares that she has relevant funding through National Institute of Neurological Disorders and Stroke (1R01 NS100859-01). Nicole Rogus-Pulia declares that she has no conflict of interest. Nathalie Rommel declares that she is a co-inventor of a relevant patent (AU2011301768 Patentee: Women’s and Children’s Health Network Incorporated). Ashli O’Rourke declares she is a Consultant for Medtronic Inc.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omari, T.I., Ciucci, M., Gozdzikowska, K. et al. High-Resolution Pharyngeal Manometry and Impedance: Protocols and Metrics—Recommendations of a High-Resolution Pharyngeal Manometry International Working Group. Dysphagia 35, 281–295 (2020). https://doi.org/10.1007/s00455-019-10023-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-019-10023-y