Abstract
The purpose of this study was to ascertain the effect of aging on muscle contractile properties associated with tongue protrusion in a rat model. Fischer 344/Brown Norway hybrid rats, ten young (9 months old) and ten old (32 months old), were used to measure protrusive contractile properties. Results showed a significant reduction in tetanic forces in the old animals. The following measures of muscle contraction were not different between age groups: mean twitch contraction force, twitch contraction time, twitch contraction half-decay time, and a calculated measure of fatigability. In conclusion, aging influenced protrusive tongue muscle contractions in a rat model such that tetanic forces were reduced. The reduction of tetanus force may parallel findings in human subjects relative to isometric tongue force generation and may be associated with age-related disorders of swallowing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Muscle atrophy is a consequence of aging and is likely a key determinant in the reductions in strength and endurance observed in senescent muscles. In the upper airway, aging is often associated with functional declines in swallowing status [1–3], phonatory ability [4, 5], and upper airway maintenance during sleep [6]. The underlying mechanisms for these observed changes with aging may be a loss of skeletal muscle mass and strength that can affect both muscle force generation and timing [7, 8]. For instance, where swallowing is concerned, elderly people tend to swallow more slowly, such that total duration of oropharyngeal swallowing is prolonged relative to younger adults [8]. Accordingly, altered strength and temporal properties of oropharyngeal muscles are likely contributors to impaired performance of critical head and neck functions in elderly people.
The tongue is a key contributor to speech, swallowing, and respiratory actions. Tongue actions during bolus preparation and swallowing are the result of muscle contraction forces in both the instrinsic muscles of the tongue and the extrinsic muscles that mediate protrusive and retrusive actions. The primary muscle for tongue protrusion is the genioglossus muscle (GG), which is innvervated by the medial branch of the hypoglossal nerve. Alterations in GG muscle function with aging may contribute to functional declines in the oral stage of the swallow.
Because motor control of the tongue is important to performance of critical head and neck functions, direct knowledge of specific age-related changes in muscular contraction properties in both retrusive and protrusive muscles is warranted. Unfortunately, the muscles of the tongue have been understudied, perhaps because of their complex architecture. While a few comprehensive studies of tongue muscle contraction properties exist [9–12], most have not examined the effects of aging specifically or have examined only retrusive tongue actions.
The purpose of this study was to determine if age-related changes were manifested in the muscle contractile properties associated with tongue protrusion in a rat model. Our hypothesis was that protrusive tongue muscle contraction forces would be diminished and temporal characteristics prolonged in old rats relative to young.
Methods
Ten adult male (9 months old, 424.5 g [SD = 37.2]) and ten old male (30 months old, 549 g [SD= 29.4]) Fischer 344/Brown Norway rats were used in this research. The median life expectancy of Fisher 344/Brown Norway rat is approximately 36 months [13].
Surgical Method
After anesthesia (ketamine 70 mg/kg; xylazine 7 mg/kg; intraperitoneal injection), the hypoglossal nerves were exposed bilaterally under microscopy with a middle incision at the anterior neck. The bifurcation of the hypoglossal nerve into the medial and lateral branches was found deep to the intersection of the lateral edge of anterior belly of digastric muscle and the edge of the myohyoid muscle. The medial branch of the hypoglossal nerve is known to supply motor innervation to the GG muscle and intrinsic muscles of the tongue, while the lateral branch is known to innervate the retrusive muscles of the tongue. Using microscissors, a 2–5-mm section of the lateral branch of the hypoglossal nerve was removed bilaterally. This section encompassed nearly the entire length of the lateral branch from the bifurcation point (within 1 mm) to the proximal point of insertion into the GG muscle. As such, while whole hypoglossal nerves were stimulated in our experiments, because of the lateral hypoglossal branch section only the medial branch was effectively stimulated.
Hypoglossal Nerve Stimulation and Muscle Contraction Recordings
Muscle contractile properties of the tongue were measured using standard physiologic testing methods routinely used in muscle physiology laboratories. Briefly, the variables of muscle contraction measured in our study were included to fully characterize tongue contraction forces (i.e., twitch and tetanic tension) and also temporal properties of muscle contraction (contraction time and half-decay time). Measures are shown in exemplar figures in the Results (Figs. 1A, 2A, and 3A).
Peak twitch force, or twitch tension (g), as described below for our study, is a measure of maximum tension generated by a muscle or a group of muscles from a single supramaximal stimulation of the motor nerve supplying that muscle. Tetanic force (i.e., fused tetanus), on the other hand, is a measure of the amount of force generated by repeated stimulations and represents a fused force signal. For a complete description of muscle function, it is important to measure both of these force variables. The fatigue ratio, as described below, is based on the change in tetanic tension levels over time and is a measure of the reduction in force found as a muscle is continuously driven to contract. This measure has been used previously in other studies that examined fatiguing characteristics of the rat tongue [10]. Force (twitch and tetanic tension) and fatigue measures are physiologically significant because they reflect the strength capabilities of the muscles being stimulated and the resistance to fatigue of these muscles.
The temporal variables of contraction time and half-decay time are measured from the twitch tension signal and are associated with how rapidly muscles contract or are able to recover from a contraction, respectively, following stimulation. Accordingly, the physiologic entity of muscle contraction speed is associated with the twitch contraction time and half-decay time. Contraction times may be altered with aging and thus it is important to measure temporal factors in studies of muscle contraction changes with aging [14]. In prior studies, tongue twitch contraction times for evoked retrusive tongue actions was prolonged in old rats when compared with young, while there was no difference in twitch half-decay times [15]. Conversely, in rat larynx (thyroarytenoid muscle), half-decay time was prolonged in old animals without a concurrent extension of twitch contraction time [16]. Accordingly, our goal was to examine these temporal elements for evoked protrusive tongue actions in old vs. young rat tongue. These temporal measures are physiologically significant because they are associated with the speed, or velocity, of muscle contraction.
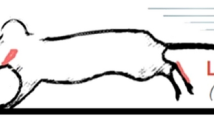
Tongue force was measured by attaching a suture to the tip of the tongue and then connecting the suture to an isometric force transducer (TRN0011, Kent Scientific, Kent, CT). The animal was placed onto a fixed stage with the tongue extended at a preload force that varied between 3 and 5 g, which is comparable to preload forces used in previous reports [3]. The fixed stage allowed manipulation of suture angle, which coincided with the contraction angle to maximize recorded forces (Fig. 4).
Muscle contractile characteristics were measured in response to stimulation of the medial branch of hypoglossal nerves bilaterally by 0.1-ms rectangular pulses at a supramaximal applied current (generally between 300 and 500 μA). Supramaximal stimulation was defined as 1.5 times the current level required to obtain maximal twitch force (A-M Systems Differential AC amplifer, model 177; A-M Systems Isolated Pulse Stimulator, model 2100; A-M Systems Analog Stimulus Amplification Unit, model 2200, Carlsborg, WA) . Bipolar Ag-AgCl hook electrodes were used. For muscle twitch, stimuli were delivered at 1 Hz. Tetanic forces were recorded using 200-ms trains with stimulus rates at 60, 80, and 100 Hz. Fatigability was measured using a 2-min fusion stimulation paradigm consisting of a 100-Hz train for 500 ms [17]. Data were acquired directly into the laboratory computer using software developed for this purpose (Aquire 1.5; Madison, WI). Twitch tension, contraction time, and half-decay time were averaged across ten trials.
The following measures were made: mean twitch contraction force, twitch contraction time (CT), twitch contraction half-decay time (HDT), tetanic force, and a calculated measure of fatigability. Mean CT was measured as the duration between the onset of stimulation and the point of 50% maximal force. HDT was the mean duration between the onset of stimulation and the point of 50% decay from peak force. Tetanic force was the maximal force of each stimulated fused wave. Fatigability was expressed as the fatigue index (FI) and was calculated as muscle force following 2 min of stimulation divided by the initial force [9]. A relatively stable 300-ms portion of both the initial force and 2-min force signals was used to calculate the FI.
Results
The old animals were significantly heavier than the adult animals (t test; t = 11.3, p < 0.0001) and weight was significantly correlated with tetanic force across groups (Spearman’s rho = 0.69; p = 0.007). Timing measures were not significantly correlated with weight. Because weight and force were correlated, force data from the old and adult groups were compared using analysis of covariance (ANCOVA) with weight as a covariate. Timing measures were compared between groups using t tests. A critical level of α = 0.05 was used to determine statistical significance. All analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC).
Twitch Contraction Force
Representative twitch force signals from one old and one adult rat are shown in Fig 1A. Although old animals generally had smaller protrusive tongue twitch forces than adult animals (Fig. 1B), the difference was not statistically significant with ANCOVA (F = 1.55, p = 0.30).
A Representative twitch contractions (g) from one old and one young animal. Contraction time (CT, ms) was measured as the duration between the onset of stimulation and the point of 50% peak force. Half-decay time (HDT, ms) was the duration between the onset of stimulation and the point of 50% decay from peak force. B Comparison of mean twitch forces (g) between old and young animals. C Comparison of mean contraction times (ms) between old and young animals. D Comparison of mean HDT (ms) between old and young animals. All error bars reflect standard deviations. There was no significant difference between age groups on any of the comparisons (p < 0.05)
Contraction Time (CT) and Half-Decay Time (HDT)
Temporal features of protrusive tongue twitch were analyzed using t tests because analysis of temporal measures did not require a weight covariate. Representative twitch contraction signals demonstrating CT and HDT measures are found in Fig. 1A. As shown in Fig. 1C and D, CT and HDT were not significantly different between age groups (CT: t = 1.45, p = 0.17; HDT: t = 0.33, p = 0.74).
Tetanic Contraction Force
Representative tetanic force signals from one old and one adult rat are shown in Fig. 2A. Fused tetanus was typically observed at stimulation frequencies of 80-100 Hz across old and adult rats. As shown in Fig. 2B, old animals evidenced significantly smaller protrusive tetanic forces compared with young with ANCOVA (F = 9.12, p = 0.002).
A Representative tetanic contractions (g) from one old and one young animal. Fusion of tetanic contraction was established at either 80 or 100 Hz. B Differences in mean tetanic force production between old and young animals was found such that old animals had significantly reduced tetanic force levels when compared with young rats (p < 0.002). Error bars = standard deviations
Fatigue Index
Representative signals used to calculate the fatigue index for old and adult animals are shown in Fig. 3A. The old and adult groups were not found to differ statistically in fatigue index using ANCOVA (Fig. 3B; F = 0.05, p = 0.95).
A Representative tetanic force signals used in the calculation of fatigue index in one old and one young rat. The larger force wave is the initial force signal, which degraded over 2 min of contraction and is represented by the smaller force signal in each figure. B Mean fatigue index was not significantly different between old and young animals (p < 0.05). Error bars = standard deviations
Discussion
We found that maximal tetanic forces for protrusive contractions of the tongue were significantly reduced in old rats. Tongue twitch forces and fatigue were not significantly different between age groups in the full cadre of animals studied. Reductions in senescent skeletal muscle forces have been reported previously in the literature [14, 18] and our findings are consistent with these reports. Furthermore, our findings parallel reports of reduced isometric tongue forces in elderly humans [19]. As such, the rat tongue presents an adequate model for the study of mechanisms underlying age-related reductions in tongue strength.
We did not find that aging influenced temporal features of protrusive tongue muscle contractile properties, as we hypothesized. Measures of fatigue also were not significantly different between age groups. Temporal features of swallowing in humans have been shown to change with aging. Specifically, increased duration of the oral portion of the swallow has been identified in elderly persons prior to the initiation of the pharyngeal phase [8, 20]. The fact that old rats, overall, were not significantly different from young rats with respect to our temporal measures is likely the result of the variability manifested within our group of old animals and the fact that we were measuring isolated protrusive contractions and not integrated lingual movements for swallowing.
While we based our methodology of examining tongue protrusion contractions in a rat model on prior studies [9–11, 21, 23], there were methodologic differences that may have resulted in larger tongue twitch and lower tetanic forces in our study than in some prevous reports. For instance, the angled suture used in our study was associated with strain gauge loading rather than unloading and may have represented both anterior and inferior evoked tongue actions, thus recording greater twitch forces. Twitch force magnitudes in our study were consistent with previous findings employing force transduction methods that loaded the strain gauge [22]. In addition, our method did not surgically isolate the GG and our stimulation paradigm likely stimulated contraction of the intrinsic muscles of the tongue (i.e., verticalis and transversus). It is possible that intrinsic muscle contraction may have effectively reduced the tongue forces recorded for tetanic force recordings as a result of induced bending. Accordingly, methodologic differences across reports must be considered when synthesizing the literature on tongue protrusion forces.
Recent studies have reported a significant negative relationship between maximum protrusive tongue force and age in humans [23] and decreased tongue blade pressures on maximal isometric pressure tasks [19, 24]. Because mixing food in the oral cavity, holding the bolus, chewing, and swallowing all require protrusive and retrusive actions of the tongue [25], reductions in tongue strength may contribute to age-related deficits in deglutition and swallowing. However, as reported by Nicosia et al. [19], reductions in isometric tongue forces may not generalize to reduced tongue forces (as measured by tongue pressures) during the swallow in elderly people. These authors have suggested that such findings may indicate a reduction in functional reserve in tongue strength that puts older people at risk for dysphagia when challanged, such as after an illness or injury [26, 27]. In this vein, elderly people may become symptomatic for swallowing impairment when the system is challenged by injury or illness because of age-related reductions in capacity to generate adequate tongue forces. It is in these situations that reductions in tongue muscle forces may become significant and contribute to manifestations of dysphagia.
Evidence of tongue movement abnormalities in persons with dysphagia has been associated with abnormalities during the oral phase of the swallow [28, 29]. Therefore, alterations in tongue muscle contractile properties may have important functional implications. There are promising data indicating a capacity for increased tongue strength with specific tongue exercise programs in young subjects [30]. There are recent preliminary data in which progressive resistance tongue exercise programs have been successfully applied to individuals with swallowing impairment [27]. As such, direct therapeutic remediation of tongue weakness may be an emerging treatment modality that warrants further study regarding putative mechanisms underlying the observed improvments in post-treatment function. Such data do not currently exist so the rat model appears useful in exploring postexercise changes in neuromuscular characteristics.
Conclusions
In conclusion, aging influenced protrusive tongue muscle contractions in a rat model such that tetanic forces were reduced. The reduction of tetanus force may parallel findings in human subjects relative to isometric tongue force generation and may be associated with age-related disorders of swallowing and respiration during sleep.
References
Doty RW, Bosma JF: An electromyographic analysis of reflex deglutition. J Neurophysiol 1956;19:44–60
Ekberg O, Feinberg MJ: Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol 1991;156:1181–1184
Schindler JS, Kelly JH: Swallowing disorders in the elderly. Laryngoscope 2002;112:589–602
Honjo I, Isshiki N: Laryngoscopic and voice characteristics of aged persons. Arch Otolaryngol 1980;106:149–150
Ramig LA, Ringel RL: Effects of physiological aging on selected acoustic characteristics of voice. J Speech Hear Res 1983;26:22–30
Mortimore IL, Bennett SP, Douglas NJ: Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res 2000;9:389–393
Doherty TJ: Aging and sarcopenia. J Appl Physiol 2003;95:1717–1727
Robbins JA, Hamilton JW, Lot GL, Kempster GB: Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 1992;103:823–829
Fuller D, Mateika JH, Fregosi RF: Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol (Lond) 1998;507:265–276
Fuller DD, Fregosi RF: Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 2000;88:2123–2130
Fuller DD, Williams JS, Janssen PL, Fregosi RF: Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol (Lond) 1999;519:601–613
McClung JR, Goldberg SJ: Functional anatomy of the hypoglossal innervated muscles of the rat tongue: A model for elongation and protrusion of the mammalian tongue. Anat Rec 2000;260:378–386
Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW: Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci 1999;54A:B492–B501
Degens H, Alway SE: Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 2003;27:339–347
Ota F, Connor NP, Konopacki R: Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol 2005;114:799–803
McMullen CA, Andrade FH: Contractile dysfunction and altered metabolic profile of the aging rat thyroarytenoid muscle. J Appl Physiol 2006;100:602–608
Burke RE, Levine DN, Tsairis P, Zajac FE: Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (Lond) 1973;234:723–748
Brown M, Taylor J, Gabriel R: Differential effectiveness of low-intensity exercise in young and old rats. J Gerontol A Biol Sci Med Sci 2003;58:B889–B894
Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J: Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci 2000;55:M634–M640
Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J: Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol 1995;268:G389–G396
Gilliam EE, Goldberg SJ: Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 1995;74:547–555
Sutlive TG, Shall MS, McClung JR, Goldberg SJ: Contractile properties of the tongue’s genioglossus muscle and motor units in the rat. Muscle Nerve 2000;23:416–425
Mortimore IL, Fiddes P, Stephens S, Douglas NJ: Tongue protrusion force and fatiguability in male and female subjects. Eur Respir J 1999;14:191–195
Robbins J, Levine R, Wood J, Roecker ED, Luschei E: Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol 1995;50:M257–M262
Miller JA: Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 2002;13:409–425
Robbins JA, Connor NP, Barczi S, Effects of Aging on Swallowing. In: Calhoun K, Eibling D, Wax M, Kost K (eds), Geriatric Otolaryngology. Taylor & Francis, New York: 2006, pp 277–291
Robbins JA, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind J: The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc 2005;53:1493–1489
Lazarus CL, Logemann JA, Pauloski BR, Colangelo LA, Kahrilas PJ, Mittal BB, Pierce M: Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope 1996;106:1157–1166
Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M: Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res 2000;43:1011–1023
Lazarus C, Logemann JA, Huang CF, Rademaker AW: Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop 2003;55:199–205
Acknowledgments
The authors gratefully acknowledge Nick Weber, Jessica Tiede, Richard Konopacki, and Dr. Glen Leverson, biostatistician, in the completion of this work. In addition, the authors thank Dr. David Fuller for sharing his expertise in the recording of tongue protrusion contractions in a rat model. This work was funded by grants from the National Institute of Deafness and Other Communication Disorders (R01DC005935 and R01DC008149).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagai, H., Russell, J.A., Jackson, M.A. et al. Effect of Aging on Tongue Protrusion Forces in Rats. Dysphagia 23, 116–121 (2008). https://doi.org/10.1007/s00455-007-9103-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-007-9103-6