Abstract
Age-related changes in muscle composition and function are often treated using exercise, including muscles of the tongue to treat swallowing impairments (dysphagia). Although tongue exercise is commonly prescribed, optimal tongue exercise doses have not been determined. The purpose of this study was to evaluate effects of varying tongue exercise frequency on tongue force, genioglossus muscle fiber size, composition and metabolism, and swallowing in a rat model. We randomized 41 old and 40 young adult Fischer 344/Brown Norway rats into one of four tongue exercise groups: 5 days/week; 3 days/week; 1 day/week; or sham. Tongue force was higher following all exercise conditions (vs sham); the 5 day/week group had the greatest change in tongue force (p < 0.001). There were no exercise effects on genioglossus (GG) fiber size or MyHC composition (p > 0.05). Significant main effects for age showed a greater proportion of Type I fibers in (p < 0.0001) and increased fiber size of IIa fibers (p = 0.026) in old. There were no significant effects of citrate synthase activity or PGC-1α expression. Significant differences were found in bolus speed and area (size), but findings were potentially influenced by variability. Our findings suggest that tongue force is influenced by exercise frequency; however, these changes were not reflected in characteristics of the GG muscle assayed in this study. Informed by findings of this study, future work in tongue dose optimization will be required to provide better scientific premise for clinical treatments in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The negative effects of aging on swallowing function are well established [1]. The estimated lifetime prevalence of impaired swallowing (dysphagia) is approximately 33% in older people [2]. Given that the population over the age of 65 is rapidly increasing [3], more individuals than ever are at risk for developing age-related dysphagia. The profile of aged swallowing is characterized by inefficiency, with longer duration of the oropharyngeal swallow [4] and increased pharyngeal residue after the swallow [5] can increase risk of penetration or aspiration events and may lead to the development of aspiration pneumonia [6]. Adequate tongue function is required for safe and efficient swallowing because tongue actions contribute to both the oral and pharyngeal phases [7]. The tongue is used to procure and form a bolus, and serves as the driving force for propelling the bolus through the pharynx into the esophagus [7]. Tongue pressures during swallowing actions are reduced in older individuals, perhaps due to changes in tongue structure and physiology [8]. Consequently, elderly people may have reduced tongue strength [9,10,11], which is associated with difficulty eating, impaired swallowing, and reduced food consumption [12]. The combined effect of these swallowing pathologies has a negative impact on quality of life and increases morbidity and mortality, and risk of malnutrition [13,14,15,16,17].
Aging is associated with sarcopenia [18], characterized by a reduction in number and size of skeletal muscle fibers [19, 20] and alterations in the composition of muscle fiber types [20,21,22,23]. Type II (fast) fibers are differentially impacted by aging and decrease in fiber size, while type I (slow) fibers are less affected [20, 23]. The causes of these age-related changes in skeletal muscle are not fully understood, but likely involve a combination of processes including decline in overall activity level, altered gene expression [24], age-related loss of motor units [25], and changes in muscle metabolism, hormones, and protein synthesis [26, 27]. These age-related shifts in muscle fiber innervation, type, size, and volume contribute to overall change in muscle contractile properties, toward a more slowly contracting and less powerful profile [18, 28,29,30,31,32]. These changes in muscular characteristics likely alter swallow function.
Mitochondrial structural changes within the muscle are also apparent with aging. Mitochondrial function is critical to maintaining an energy supply (adenosine triphosphate; ATP) to contracting muscles [33], and it is well established that aging negatively impacts mitochondrial structure and function [34,35,36], resulting in decreased ATP production for cellular use [33, 37,38,39]. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcription coactivator [40] that signals a cascade of gene expression resulting in proliferation of mitochondrial content, and other effects [40,41,42,43]. Thus, PGC-1α protein expression is an upstream indicator of mitochondrial biogenesis and is used as a marker of mitochondrial content [44,45,46,47,48]. Another protein, citrate synthase, is a downstream marker of mitochondrial function related to genesis of ATP [43, 46, 49,50,51]. Due to the specific function and exclusive location of citrate synthase within the mitochondria, citrate synthase enzyme function reflects overall mitochondrial respiratory oxidative capacity [52, 53]. Age-related degradation in mitochondrial biogenesis and function represent putative mechanisms for muscular capacity in cranial muscles that have not been systematically explored in dysphagia research.
Tongue exercise has been used to treat dysphagia across multiple etiologies with positive results [54,55,56,57,58,59], with increases tongue muscle volume and strength with associated improvements in swallowing function [17, 58, 60]. While tongue exercise is used frequently in dysphagia treatment with geriatric populations, it is not well understood how to optimize tongue exercise protocols because the underlying biological mechanisms of these treatments are unknown. Further, the appropriate dose of tongue exercise to achieve optimal results has not been determined [61, 62].
It is well established that exercise dose is an important component of muscular conditioning and contributes to training outcomes [63]. However, there is not a consensus on appropriate dosing for tongue exercise as a treatment of dysphagia [61]. As a result, there are a wide range of recommended doses currently used for tongue exercise [54, 56, 58, 64, 65]. Studies using tongue exercise regimens vary from 3 times/day for 3 days/week [54, 58, 64] to as many as 7x/day, 7 days/week [65, 66]. An understanding of the manner in which tongue exercise dose affects muscle structure and function would be beneficial in optimizing treatment plans [61, 67]. It is not necessarily the case that higher exercise doses result in greater muscle strength and greater improvements in patient outcomes. In some cases, over prescription could result in diminishing returns or even injury [68]. Higher doses of exercise could also lead to poor adherence with treatment recommendations and potentially limit treatment benefits as a result. Recommendations for tongue exercise programs should be based on strong scientific criteria.
While our laboratory has demonstrated the utility of an aging rat model to study tongue exercise as a treatment for dysphagia at the biological level [69,70,71,72,73,74,75], we have not explored the effects of varying tongue exercise dose on biological, metabolic, and functional outcomes. Incorporation of different exercise doses into research studies will address clinically relevant gaps in knowledge and will allow for discovery of the association among biological assays and behavioral outcome measures. Therefore, the purpose of this study was to: (i) quantify effects of altering tongue exercise dose (frequency; # days/week) on tongue force after an 8-week exercise program; (ii) examine effects of tongue exercise dose on biological composition of the genioglossus muscle to determine how differential dose prescriptions alter muscle fiber size and composition; (iii) quantify the impact of increasing tongue exercise frequency on mitochondrial content and function as measured by PGC-1α expression and citrate synthase activity in the genioglossus muscle; and (iv) determine dose-related effects of tongue exercise on swallowing physiology as measured through videofluoroscopic swallow studies. Given the known changes to occur with again muscle fiber biology and physiology, we opted to study tongue exercise frequency effects in both young adult and old rats to see if age differentially impacted any of our selected outcome measures, as these data can serve to inform future studies in human participants.
We hypothesized that greater frequency of tongue exercise would result in greater gains in tongue force, a higher proportion and size of MyHC I and IIa fibers, increased signaling for mitochondrial biogenesis by elevated PGC-1α expression with concomitant increases in citrate synthase activity, increased swallow speed and more efficient mastication of the bolus (increased mastication rate).
Materials and Methods
Use of live animals was performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition; 2011) and approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee.
Animals
Eighty-one Fischer 344/Brown Norway rats were obtained from the National Institute on Aging rat colony (Charles Rivers Laboratories; Wilmington, MA). At study completion, 41 rats were approximately 32 months old (old group) and 40 rats were approximately 9 months old (young adult group). Upon arrival, young adult and old rats were randomly assigned to one of four groups: (1) Sham control; (2) One day/week tongue exercise; (3) Three days/week tongue exercise; and, (4) Five days/week tongue exercise (Fig. 1). Power calculations indicated that at least 10 rats per group allowed for detection of tongue strength differences across exercise conditions (α = 0.05, power = 90%, effect size d > 0.8) [76].
Preparation
Rats were housed in pairs in polycarbonate cages in a humidity- and temperature-controlled facility with reverse light–dark cycling (12:12 h) to maximize levels of activity during exercise and testing. Rats were acclimated to these conditions with food and water provided ad libitum and with handling by research personnel for at least 10 days prior to testing.
Videofluoroscopy
After the handling acclimation period, rats were slowly food-restricted over 5 days to encourage consumption of barium-infused substances during videofluoroscopic swallow studies (VFSS). This food restriction protocol involved reducing access to standard rat chow from ad libitum to only 3 h per day over 4 days. On videofluoroscopy testing days, rats were transported to the testing facility, given Wanda’s ® Barium Cookies (Wanda’s Barium Cookie LLC; Calumet, MI), and imaged in a standard C-arm fluoroscopy suite (Artis Zee—Siemens; Frankfurt, Germany) with an acquisition rate of 30 frames per second. The rat enclosure was positioned to allow for imaging from a lateral view. Rats moved freely within the enclosure during image acquisition. Up to 10 swallows were imaged and recorded per session. Swallow studies were conducted 3 days prior to initiation of a water restriction protocol (described below) and 3 days after completion of the tongue exercise and testing sequence (described below) to allow for pre-post comparison of swallow physiology.
Tongue Exercise
Tongue exercise time points are represented in Fig. 2.
Tongue exercise schedule. Each box represents a single day in the acclimation and tongue exercise protocol. After 7 days of acclimation, rats underwent Maximum Voluntary Tongue Force (MVTF) testing to determine maximum tongue forces prior to training. This value was used to calculate daily threshold values during exercise and was re-evaluated at the end of Week 4 for the exercise animals only (5-day, 3 day, 1 day/week groups only). Exercise days occurred Monday through Friday, where rats entered the Tongue Exercise Apparatus and completed either sham exercise (less than 20 presses at 2 mN force threshold) or tongue exercise (average 100 presses at set threshold for that training week). Exercise days for each group are indicated in the figure. At the end of each exercise day Monday–Friday, all rats received 3 h of water ad libitum in the watering cages
Methods related to tongue exercise have been described in detail previously [69, 71, 73], and are reviewed briefly here. Prior to acclimation to the tongue exercise apparatus (Fig. 3), rats were placed on a 10-day water restriction protocol, where access to water was incremented down from full access to only 3 h of access per day [77]. During this water restriction protocol, rats were placed in individual polycarbonate cages, similar to the tongue exercise enclosure, with dishes of water placed in an area analogous to the location of the operandum in the tongue exercise enclosure (see Fig. 3). Once rats received only 3 h of water/day for 5 consecutive days before acclimation to the tongue exercise apparatus began. During acclimation, rats were classically conditioned to press the tongue against a disk for a water reward on a variable 1:5 reinforcement schedule. Tongue force was measured by a force transducer (Sensotec load cell, 0–245.17 mN). Force transducer output was collected by custom-designed acquisition software (Matrix Product Development; Cottage Grove, WI) with a precision of 2 mN and a 200 Hz sampling rate.

Adapted from [69]
Exercise apparatus and watering cages. Watering cages are used to prime rats for tongue exercise training using classical conditioning principles, providing a reinforcement (water) in the same location as in the training apparatus. The Training Apparatus dispenses water onto the disk when the rat presses with adequate force as measured through the force transducer.
After 7 days of acclimation to the exercise apparatus, where only a low force was required for water to dispense (2 mN), rats were challenged to press the disk at increasing force levels by incrementing the threshold for receipt of a water reward. The average of the greatest 10 tongue presses across 3 consecutive days represented the Maximum Voluntary Tongue Force (MVTF) measurement for both baseline and post-exercise time points. The sham control group received MVTF testing only at baseline and after 8 weeks of the sham control condition. For exercise groups, reward thresholds for each individual rat across the 8 weeks of tongue exercise were calculated as a percentage of that rat’s MVTF. For weeks 1 and 2, reward thresholds were set to 50% of MVTF, and were then set at 60% for weeks 3 and 4. MVTF was re-determined for tongue exercise groups only at the end of week 4. Following this re-assessment, reward thresholds were set to 70% of the revised MVTF value for weeks 5 and 6, and finally at 80% for weeks 7 and 8.
On tongue exercise days (Fig. 2) rats assigned to the 1, 3, or 5 day/week tongue exercise groups entered the tongue exercise chamber and pressed the disk with the tongue approximately 100 times at or above the predetermined threshold to obtain a water reward. Sham control rats entered the exercise apparatus 5 days/week but were allowed to press the disk < 20 times with no threshold increase over the minimal level (2 mN) to demonstrate learning the task but without participating in exercise. On non-exercise days, the 1 and 3 day/week rats entered the exercise apparatus and followed the same protocol as sham control animals to maintain performance of the task. All rats received 3 h of access to water after tongue exercise or sham control conditions on each treatment day. Rats did not enter tongue exercise apparatus on weekend days, but received access to water 3 h on each weekend day.
Tongue Exercise Dose for Exercise Groups
During 8 weeks of tongue exercise (duration), 3 exercise conditions dictated the number of days per week of exercise (frequency). The targeted number of tongue presses per session (repetitions) was held at approximately 100 at the predetermined threshold (intensity). That is, on exercise days, rats in 1 day, 3 days, and 5 days/week exercise groups pressed the disk approximately 100 times per session above the predetermined force threshold for that exercise session. However, due to the behavioral nature of the task, some rats did not achieve the 100-press criterion on some days. To confirm actual dose of exercise administered across all rats, we monitored and recorded daily averages for force per session (intensity) and number of presses per session (repetitions). To calculate these daily averages, we included presses that registered at or above the designated force threshold for an individual exercise session.
Muscle Tissue Harvest
Within 72 h of final VFSS, rats were euthanized with Beuthanasia intraperitoneal injection (0.2 cc) after anesthesia with isoflurane. Paired GG muscles (left and right side) were removed and randomly assigned to either to whole muscle snap freezing in liquid nitrogen for bioenergetic assays (citrate synthase and western blotting) or whole muscle freezing in optimum cutting temperature (OCT) fixing solution and stored at − 80 °C for later sectioning for immunohistochemistry (IHC). Extensor digitorum longus (EDL) and soleus (SO) muscles were harvested at random from one animal per exercise/sham group to serve as biological controls for IHC (see below).
Immunohistochemistry of Genioglossus Muscle
The IHC staining protocol was performed over three consecutive days for sectioning, staining, and imaging.
Sectioning
Using a cryostat (Leica CM 1850; Meyer Instruments, Houston, TX), GG muscles were cut into 10 µm cross-sections and mounted on slides at − 20 °C. To stain for all 4 MyHC isoforms (I, IIx, IIb, IIa), two serial sections were taken from the midbelly of each GG to avoid regional differences that have been previously found in this muscle [74], resulting in 2 slides per rat.
Staining
The staining protocol has been used previously in our laboratory [78] and is validated in the literature [79,80,81]. After sectioning, slides were stored at − 20 °C overnight until performing the staining protocol the following day. The first slide was stained for laminin, MyHC I, IIa, IIb and the second slide was stained for laminin MyHC IIa and IIx (Fig. 4a–c). Different primary antibodies were applied to each slide to stain for different MyHC isoforms (see Table 1). Two slides are required because secondary antibodies for MyHC isoforms IIx and IIb both illuminate red when imaged (Table 1) [78]. MyHC Type IIa isoform was stained on both sections. Sections from EDL and SO muscles were used with each staining batch to serve as controls and validation for staining protocol (Fig. 4c, d): EDL muscles are majority (> 50%) Type IIb fibers and SO are majority (> 50%) Type I [82]. These were selected as biological controls given that relative fiber composition for these muscles have been well studied in the limb literature.
Representative example from 1 genioglossus (GG) muscle: TIFF files for Semi-Automatic Muscle Analysis (SMASH) after ImageJ processing. a Blue outlines laminin, Red indicates MyHC Type I isoform, b Blue outlines fiber laminin, Green indicates MyHC Type IIa isoform, Red indicates MyHC Type IIb isoform, c Blue outlines fiber laminin, Green indicates MyHC Type IIa isoform, Red indicates MyHC IIx isoform. Representative examples for staining validation from 1 extensor digitorus longum (EDL) muscle demonstrating Type IIb in Red and 1 soleus (SO) muscle demonstrating Type I in Red
Prior to staining, slides were first fixed in cold acetone for 10 min followed by phosphate buffered saline (PBS) washes (2×, 2 min each). Following washes, 10% normal goat serum (NGS) was applied as a blocking solution for 30–45 min. Primary antibodies were applied to each group of slides for 1 h and 15 min at room temperature (see Table 1). Following primary incubation, slides were blotted and rinsed with PBS (3×, 5 min each) prior to secondary body application (see Table 1). Slides were incubated in secondary antibody for 45 min, covered, at room temperature. Following secondary application, slides were washed a final time in PBS (3×, 5 min each) and left to air dry before cover slipping with ProLong Anti-Fade without DAPI (Invitrogen Life Technologies, Eugene, OR). Slides were stored at room temperature overnight before imaging the following day.
Imaging
All slides were imaged on the third day with an Olympus BX53 Upright Microscope (Center Valley, PA) using Cell Sense Dimension (117) acquisition software. GG sections were imaged at 20 × resolution to allow capture of the entire section. Prior to image analysis, files are prepared in ImageJ, converting. VSI to.TIFF files. Relative immunofluorescence was converted to RBG, which was used by the Semi-Automatic Muscle Analysis (SMASH) program [83] for MATLAB analysis. A custom ImageJ (National Institutes of Health, LOCI University of Wisconsin) script was used to convert colors on images to allow us to distinguish laminin from MyHC Type I fibers (Fig. 4a), measurement of MyHC IIa and IIb fibers (Fig. 4b), and measurement of MyHC IIa and IIx fibers (Fig. 4c).
Analysis
Files were uploaded into MATLAB (MathWorks, Natick, MA) using the Semi-Automatic Muscle Analysis (SMASH) program [83] to determine fiber cross sectional area (CSA) by proportion of fibers staining positive for each MyHC isoform Type (I, IIx, IIb, IIa) based on signal of staining intensity—threshold values are set to capture all positively stained fibers based on a histogram provided in SMASH of staining intensities. Measurements for CSA were determined based on the pixel size of the 20 × objective of the Olympus Scope (0.5119 µm/pixel). Tissue sections were excluded (n = 21) if there was freezer artifact or if the section contained a majority of longitudinal fibers rather than cross sectioned fibers.
Homogenization
GG muscles that were snap frozen during final harvest for bioenergetic assays were homogenized in CelLytic™ MT buffer (Sigma Aldrich, St. Louis, MO, product code C 3228) with EDTA, protease, and phosphatase inhibitors added (1:10). Following homogenization with scissors, samples were sonicated and spun at room temperature for 10 min at 20,000 rcf. Supernatant was transferred to a separate tube and stored in aliquots at − 80 °C prior to analysis. Total protein was quantified using a Bradford Protein Assay (Thermo Fisher Scientific, Maltham, MA, #23200) prior to freezing aliquots.
Citrate Synthase Activity Assay
Citrate synthase activity was quantified using a Citrate Synthase Assay Kit (Sigma Aldrich, St. Louis, MO, product code CS0720). The sample was loaded into a 96 well plate at 8 μg/well and the kinetic reaction between acetyl coenzyme A and oxaloacetic acid (OAA) is quantified using spectrophotometry at 412 nm with an Eon microplate spectrophotometer (BioTek Instruments Inc, Winooski, VT) using Gen5™ (BioTek Instruments Inc, Winooski, VT) collection and analysis software. Control wells were included (sample without OAA added) and a positive control of citrate synthase (included with kit) was used to ensure quality for each batch of reactions measured.
Western Blotting for Expression of PGC-1α
Western blotting was used to detect expression level of PGC-1α in GG muscle homogenates. Samples were diluted 1:1 with XT sample buffer (Bio-Rad, Hercules, CA), heated for 5 min at 95 °C and loaded into Criterion XT 4–12% Bis–Tris gels (Bio-Rad, Hercules, CA) with 22.5 ug/well concentration and run for 60 min in XT MOPS (Bio-Rad, Hercules, CA) running buffer using the Bio-Rad Gel Electrophoresis system at 200 V constant current. Gels were then transferred to membranes at 4 °C in Townbin Transfer Buffer using the Bio-Rad Transfer Tank system for 40 min at 100 V constant current. Due to age-related variation of expression in common housekeeping proteins such as GADPH and β-actin [84], we opted to use internal loading controls to normalize analysis across western blots. Accordingly, skeletal muscle lysate from mouse (Abcam, Cambridge, UK, Ab29711) served as our internal loading control. We completed total reversible staining of proteins after transfer using Memcode Reversible Protein Stain and Destain Reagent (Pierce™ Reversible Protein Stain Kit for nitrocellulose membranes, Thermo Fisher Scientific, Maltham, MA) [85, 86]. Blots were then imaged for total protein (25 ms exposure, UVP ChemiDoc-IT2 Imager, Analytik Jena, Jena, Germany) and analyzed using VisionWorks software (Analytik Jena, Jena, Germany). After imaging, MemCode Stain Eraser was used to clear bands from membrane prior to blocking for one hour at room temperature (filtered 5% Milk in TBS-T).
After blocking, membranes were incubated in primary antibody for 14 h at 4 °C (Anti-PGC-1α antibody N-terminal, ab191838, Abcam, Cambridge, UK; 1:500 dilution in filtered 5% Milk in TBS-T). Blots were removed from 4° one hour prior to TBS-T washes (5 min, 5×). Membranes were then incubated in secondary antibody for one hour at room temperature (Donkey anti-Rabbit IgG HRP, Thermo Fisher Scientific, Maltham, MA SA1-200; 1:1000 dilution in TBS-T). Following TBS-T washes (5 min, 5×), blots were incubated in SuperSignal™ West Pico Plus Chemiluminescent Substrate (Thermo Scientific, Maltham, MA) for 5 min prior to detection. Blots were then detected using UVP ChemiDoc-IT2 Imager using the Total Dynamic Exposure setting capturing 20 frames over 10 min.
Files were stored and then uploaded into VisionWorks (Analytik Jena, Jena, Germany) for final quantification of PGC-1α expression as measured by Mean Density of band intensity. Total Protein Assay from Memcode Reversible Protein Stain was used to normalize relative expression within a blot; skeletal muscle tissue control (Abcam, Cambridge, UK, ab29711) was used to normalize expression across blots. PGC-1α expression was quantified at the 130 kD band, per instructions for this primary antibody (Anti-PGC-1α antibody N-terminal, ab191838, Abcam, Cambridge, UK).
Analysis of Videofluoroscopic Swallow Studies
Videofluoroscopic swallow studies were blinded and analyzed. To control for swallow-to-swallow variation, 3 swallows per rat were analyzed using ImageJ for average bolus speed, bolus area, and mastication rate. Bolus speed was calculated as change in distance (pixels/mm) over time (frame rate, 30 fps) using the “multi-point” tool in ImageJ to track the head of the bolus from the initiation of the swallow (first frame when bolus exits oral cavity) through the upper esophageal sphincter. Bolus size (pixels/mm) was measured using the “freehand selections” tool in ImageJ by outlining the bolus. Mastication was measured across 5 full cycles, rate was determined using number of frames/5 cycles of mastication. Because rats were free to move about the cage, we excluded rats from analysis if three swallows were not in a full lateral view to allow measurement of each variable (bolus speed, bolus area, mastication rate). Magnification was used during recording of each fluoroscopy session. Appropriate calibration factors were applied to allow accurate measurements. Both intra- and inter-rater reliability were assessed. Reliability was assessed by remeasurement of bolus speed, bolus area, and mastication rate for 10% of the measurements by the primary investigator and by a second investigator. An intraclass correlation coefficient (ICC) of > 0.95 was achieved for both intra- and inter-rater reliability.
Statistical Analyses
All statistical analyses were performed in SPSS (IBM Software) using two-way analyses of variance (age x exercise) with a critical α of ≤ 0.05 for all comparisons (main and interaction effects). If appropriate, post hoc tests were conducted using a Tukey’s correction to account for multiple comparisons. The following variables were assessed: number of presses/session, average force per session (mN), change in maximum voluntary tongue force (MVTF; mN), fiber size (CSA, μm2), MyHC isoform composition (%), citrate synthase activity (nmol/mg/min), relative expression of PGC-1α (arbitrary units), change in bolus speed (mm/s), change in bolus area (mm2), and change in mastication rate (cycles/s).
Results
Exercise Dose (Intensity, Repetitions)
We tracked tongue presses at or above the set force threshold for each training day, per rat, to measure dose-delivery of intensity. Data for average force above threshold (mN) per exercise group are presented in Fig. 5. Overall, average daily forces were very close to threshold with a range of 10.2–12.4 mN between the threshold set and the daily average force across groups. There were significant differences in average daily force between 5 day/week exercise group and 1 day/week group. The 5 day/week rat group had higher daily tongue forces, on average, than 1 day/week group (12 mN vs 10.4 mN, respectively, p = 0.047). We also tracked average number of presses per day with a target of 100 repetitions per day. Across groups, there were no significant differences in average number of presses per day (repetitions; p > 0.05 for all comparisons).
Average difference in daily force threshold. Error bars represent standard deviation. The average difference between the assigned force threshold (% maximum voluntary tongue force) and the actual force threshold achieved (tongue force during exercise) per exercise day are displayed. Average force per session across groups ranged between 10.2 and 12.4 mN above threshold. 5 day/week rats did have slightly higher average daily forces (above threshold) as compared to the 1 day/week exercise group (p = 0.047). Y young adult, O old; 1 = 1 day/week exercise; 3 = 3 days/week exercise; 5 = 5 days/week exercise
Tongue Force (MVTF)
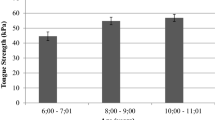
There was a significant main effect for tongue exercise condition on change in MVTF (F3,73 = 45.271, p < 0.0001), but there was not a significant main effect for age (F1,73 = 3.306, p = 0.295) and no significant interaction effect (F3,73 = 0.508, p = 0.678). Depicted in Fig. 6, the 5 days/week exercise condition had the greatest change in MVTF after 8 weeks of tongue exercise (p < 0.001). However, change in tongue force in 1 day/week and 3 day/week exercise conditions was significantly greater than the control (sham) group (p < 0.0001). There were not significant differences for change in MVTF for 1 versus 3 days/week of exercise (p = 0.329). Of note, there were no significant main or interaction effects for age or exercise condition for MVTF measured at baseline, meaning all groups had equal tongue force measures at baseline (age F1,73 = 0.621, p = 0.433; condition F3,73 = 0.726, p = 0.540; interaction F3,73 = 1.181, p = 0.323).
Change in Maximum Voluntary Tongue Force (MVTF) after 8-weeks of tongue exercise at different dose conditions (sham control, 1 day/week, 3 days/week, and 5 days/week of exercise). There was not a significant main effect for age (F1,73 = 3.306, p = 0.295), so age conditions are combined here. There was a significant main effect for exercise condition (F3,73 = 45.271, p < 0.0001): 5 days/week of exercise had the greatest increase in MVTF. Both 1 and 3 days/week of exercise had greater increases in MVTF than the sham control condition, but less than 5 days/week of exercise
Immunohistochemistry
All fiber size (CSA, um2) and MyHC isoform content statistical values are represented in Table 2. Significant main effects for age showed a greater percentage of MyHC Type I fibers in old than in the young adult group (F1,54 = 44.927, p < 0.0001), and a greater proportion of MyHC Type IIb fibers in the young adult group (F1,54 = 13.467, p = 0.001; Fig. 7). Fiber size (CSA, μm2) by MyHC isoform also showed a significant main effect for age in MyHC Type IIa isoform (F1,54 = 5.242, p = 0.026), with larger IIa fibers in the old than in young adult group (Fig. 7). There were no significant main effects for exercise and no significant interaction effects (see Table 2).
MyHC isoform composition (%) and fiber size (CSA). Exercise groups are collapsed here by age, since there was no exercise effect across all MyHC Fiber types. There were significant main effects for age: (1) Increased percentage MyHC Type I in old (F1,54 = 44.927, p < 0.0001); (2) Increased percentage of MyHC Type IIb in young adult (F1,54 = 13.467, p = 0.001); (3) Fiber size in old MyHC Type IIa fibers was greater than young adult (F1,54 = 5.242, p = 0.026)
Citrate Synthase Activity
There were no significant main effects for age (F1,72 = 0.135, p = 0.714) or exercise (F3,71 = 0.386, p = 0.763) and no significant interaction effects (F3,72 = 0.118, p = 0.949) in citrate synthase activity.
Relative Expression of PGC-1α
There were no significant main effects for age (F1,72 = 2.348, p = 0.13) or exercise (F3,71 = 0.207, p = 0.891) and no significant interaction effects (F1,72 = 0.087, p = 0.967) in PGC-1α expression.
Swallowing Characteristics
Videofluoroscopic swallow study analysis for bolus speed, bolus size, and mastication rate are represented in Fig. 8 with sample size values ranging from 6 to 11 rats per group. We found a significant interaction effect between age and exercise (F3,66 = 6.007, p = 0.001) for change in bolus speed (Fig. 8). Post hoc comparisons revealed that the 5 day/week old group had significantly faster bolus speeds after exercise than the 3 day/week old group (p = 0.004).
Change in bolus speed, bolus area, and mastication rate after tongue exercise. For change in bolus speed, there was an interaction effect (F3,66 = 6.007, p = 0.001), where old 5 day/week rats had faster speeds after tongue training than old 3 day/week rats (p = 0.004). However, there was a pre-existing difference between these groups at baseline (3 day/week old group > bolus speed at baseline compared with 5 day/week old group p = 0.026). Change in bolus area after tongue exercise showed an interaction effect (F3,69 = 3.02, p = 0.036), 5 day/week old animals had larger boluses sizes after tongue exercise as compared to young adult 5 day/week. Change in mastication rate after tongue exercise showed no main effects for age, exercise, or interaction effects (F1,60 = 1.529, p = 0.221; F3,60 = 0.302, p = 0.824; F3,60 = 1.273, p = 0.292, respectively). Y young adult; O old; C Sham control; 1 = 1 day/week exercise; 3 = 3 days/week exercise; 5 = 5 days/week exercise
However, at baseline, the 5 day/week old group was also significantly different from the 3 day/week old group (p = 0.026). Thus, we interpret these findings with caution. For change in bolus area (Fig. 8), we also found a significant interaction effect between age and exercise condition (F3,69 = 3.02, p = 0.036). Post hoc comparisons revealed that the 5 day/week old group had significantly greater bolus areas than 5 day/week young adult group (p = 0.045). Mastication rate (Fig. 8) showed no significant main effects for age (F1,60 = 1.529, p = 0.221) or exercise (F3,60 = 0.302, p = 0.824) and no significant interaction effects (F3,60 = 1.273, p = 0.292).
Discussion
We hypothesized that increasing tongue exercise frequency would result in concomitant increases in tongue force, a greater proportion and size of more slowly contracting and fatigue-resistant muscle fibers (Type I and IIa MyHC isoforms) and increased signaling for mitochondrial biogenesis with related increases in mitochondrial activity. We also hypothesized that increased exercise frequency would result in changes in swallowing characteristics, including speed and efficiency. Our hypotheses were supported in part by our data. We observed robust and significant increases in tongue force with increased frequency of exercise. However, these changes were not reflected in biological assays of the genioglossus muscle. In addition, we did not find significant differences in swallowing characteristics as a function of exercise frequency. Negative findings in this study may be related to our exercise task, choice of assays, and variability in rat behavior during swallow studies.
Tongue Force
We hypothesized that increasing frequency of tongue exercise would increase tongue force, and our findings supported this hypothesis. The highest tongue exercise frequency of 5 days/week resulted in the greatest increase in tongue force. The 1 and 3 days/week exercise groups also had significantly greater tongue forces than the sham control group, suggesting that the lowest dose, 1 day/week of tongue exercise, was sufficient to significantly increase tongue force, but not to the same extent as 5 days/week. Previous studies using this rat model have also shown increases in tongue forces following 5 days/week of exercise [69, 71, 73], but this is the first study to show that smaller doses of exercise (1 and 3 days) are also associated with significantly increased tongue force versus control. No significant difference in tongue force after 1 versus 3 days/week of tongue exercise contrasts with findings from the limb literature, where exercise at 3 days/week results in greater gains than 1 day/week [87, 88]. This further supports the premise that tongue muscles must be explored directly, and findings from the limb may not reflect mechanisms present in the cranial motor system. Tongue exercise dosing characteristics must be studied directly to better understand dose–response in this unique group of muscles.
Muscle Fiber Biology and Metabolism
We also hypothesized that increasing frequency of tongue exercise would result in a higher proportion and size of Type I and IIa muscle fibers, as identified by staining of MyHC isoforms. However, these hypotheses were not supported by our data. We did not see a significant effect of tongue exercise in genioglossus muscle fiber size or MyHC composition at any tongue exercise dose. This is in contrast with a previous finding from our laboratory that showed a shift toward a greater proportion of MyHC I fibers with tongue exercise using whole muscle homogenate in SDS-PAGE gel assays [71]. One explanation for this might be that we only examined changes in the GG midbelly in this study when there are known regional differences within the GG in proportion of MyHC isoforms [74]. Accordingly, there could be exercise effects in anterior or posterior portions of the GG not discovered here that could be examined in a future study. Another consideration is that other tongue muscles (intrinsic—superior longitudinal, inferior longitudinal, transverse, verticalis; other extrinsic—hyoglossus, styloglossus) could be contributing to changes in tongue force after exercise. These muscles have different activation characteristics than the GG and may have been differentially affected during our protrusive tongue exercise task [89, 90]. Examining tongue exercise effects on these muscles in future studies would allow discovery of potential changes in muscle fiber biology or metabolism across different exercise doses and would provide insight on how tongue exercise may impact the synergy of tongue muscle activity during the swallow.
This study also showed that there was an age-related increase in proportion of Type I and size of Type IIa muscle fibers in the old group, which is consistent with previous work in our laboratory [71, 74]. This demonstrated a shift in old rat GG toward a slower contracting, fatigue-resistant profile. These findings are also supported by previous studies of aged tongue contractile properties, showing that fatigue was not increased with age but that the tongue had slower contraction times to reach maximal force generation [91]. This shift toward a more slowly contracting fatigue-resistant profile with age may be a compensatory change to allow for increased endurance of these muscles during critical motor activities, such as maintenance of airway patency, oral processing, and swallowing throughout a meal.
Another potential explanation for lack of identification of biological mechanisms for dose-related changes in tongue force after exercise could be that our tongue exercise task was not sufficiently rigorous to induce changes in muscle fiber properties. The genioglossus muscle is very active during a multitude of tasks including airway patency [92] and speech [93], in addition to deglutition [94]. Given this high level of activity in this particular muscle, our tongue exercise task may not have induced activity levels over and above routine muscle activation levels and thus failed to induce changes in fiber type or size [95,96,97]. Increasing number of repetitions or intensity (force threshold) of the tongue exercise task may create more of a challenge to signal for changes in muscle biology of the GG. Examination of biological variables in other extrinsic and intrinsic tongue muscles may also clarify the mechanisms responsible for dose-related increased in tongue force.
Increasing tongue exercise frequency also did not significantly increase PGC-1α expression or citrate synthase activity. Thus, mitochondrial content and function, as measured by these specific assays, do not appear to be mechanistic candidates for alterations in maximum tongue force with dose observed in this study. To compare these findings with the literature, we must clarify whether the tongue exercise program used in this study is considered an endurance, resistance, or a combination exercise program. This distinction is important because previous studies of mitochondrial changes with exercise have reported findings dependent on exercise type. Based on the definitions provided by Cartee and colleagues [98], our task makes use of both high repetitions (endurance) and high resistance (strength), given that rats are challenged to press the disk 100 times per session at increasing force thresholds (60–80% of max). Most resistance exercise programs only require 12 repetitions max (per set) [43], making our 100 presses in the “high-repetition” range. As such, the tongue exercise approach used in this study may be more similar to a combination of endurance and resistance exercise.
Studies examining mitochondrial adaptation in combination exercise programs are few, and findings are inconsistent. One study found that PGC-1α expression was almost 2 × higher after concurrent exercise than with endurance exercise alone [99]. Another study concluded that resistance exercise inhibited mitochondrial biogenesis when added after endurance (aerobic) exercise [100]. Given the few studies looking at concurrent exercise and the mixed findings it is difficult to compare our findings to those in the literature base. More conclusive findings have been reported for endurance and resistance exercise alone. Endurance exercise has been shown to increase mitochondrial content and function [101, 102], while resistance exercise does not increase signaling for mitochondrial biogenesis [103, 104]. Resistance exercise is also known to have little to no effect on citrate synthase activity [105,106,107,108,109]. Thus, findings in our study are more consistent with resistance exercise effects on mitochondria found in the limb literature, suggesting that perhaps our task is more resistance-based when it comes to mitochondrial remodeling.
In this study, we assayed for changes in muscle metabolism as measured by markers in mitochondrial content and function. It is possible that other markers of mitochondria were affected by this task that were not measured in this study, such as O2 consumption [110]. This task may also be more similar to resistance exercise than an endurance exercise task. Given that resistance exercise is generally thought to have less robust changes in upregulation of mitochondrial production, this may explain why we did not see changes in these upstream and downstream markers. However, if we changed the task to require a more endurance like task (e.g., 200 repetitions at a lower force threshold) or by inducing fatigue by stimulation of the hypoglossal nerve, we may see change in these mitochondrial assays.
We showed that 5 day/week exercise rats had the greatest change in tongue force from baseline, but that 1 and 3 day/week exercise rats had greater changes in force than controls. While all rats in this study learned the exercise task, the 1, 3 and 5 day/week animals had the opportunity to learn a more complex task that required pressing the disk with increasing force over time to receive a water reward. Control rats were not challenged to press the disk at increased intensity and pressed at a low threshold for fewer repetitions. Perhaps increasing the intensity throughout 8 weeks for exercise rats required a more skilled behavioral response to continue to receive the water reward. There is evidence to support this, showing that learning skilled tasks results in differential organization of the motor cortex as compared to unskilled tasks [111]. On the other hand, repeatedly performing a task is necessary for learning any motor skill or behavior [112]. Motor learning literature has emphasized the need for repeating a task over time to allow for long-term gains in task performance [113]. Our findings are supported by this given that 5 day/week group that performed this task with the greatest frequency of all groups, had the greatest gains in tongue force task performance.
Swallowing Characteristics
Our hypothesis that increasing frequency of tongue exercise would result in greater improvements in swallowing speed and efficiency was not supported by our data. Although statistical findings showed a significant difference between two groups (O3 vs O5) in measures of bolus speed, we are interpreting these results with caution given the large amount of variability across groups and the significant differences between groups at baseline. We also found no age or exercise effects on mastication rate, which contrasts our previous findings using a pasta biting task that showed more cycles of mastication in old rats than in young adult [110].
There are several possible explanations for these findings. First, differences in food consistency (pasta vs cookie) may account for these disparate findings. This was the first study in our laboratory to use a cookie texture during VFSS as compared to previously studied peanut butter. This difference in texture may have resulted in more variable movements during imaging because rats had greater freedom to move about the cage when handling their cookies, rather than licking peanut butter off of a fixed stage at the front of the cage. Future comparisons of swallowing characteristics across different food textures would shed more light on our findings of high variability with cookie.
An alternative interpretation of these findings could relate to tongue strength and swallowing function at baseline: our analyses demonstrated no significant difference in lingual strength between old and young adult rats at baseline. That is, rats in any exercise condition (young adult or old) did not necessarily have a deficit in lingual or swallowing function at baseline, which could explain the lack of measurable change in swallowing function as a result of tongue exercise.
One statistically significant finding that cannot be explained by a difference at baseline is bolus area between the old 5 day/week exercise group and young adult 5 day/week exercise group. After 8 weeks of exercise training, the old rat exercise group had larger bolus areas than their young adult counterparts, but no other group differences. Older rats were significantly larger than young adult rats in the 5 day/week exercise groups (p = 0.008), which may partially explain why the older rats had larger bolus areas. However, it is possible that this is an incidental finding given the wide range of variability among the fluoroscopic swallow study measurements.
Limitations
We acknowledge several limitations in this work. First, while we attempted to control the intensity at which rats pressed the disk on training days by setting force threshold, we could not control how hard rats pressed the disk. As a result, the 5 day/week exercise rats pressed on average higher than their force threshold as compared to the 1 day/week rats. This is an inherent challenge with animal behavior that we acknowledge as a limitation. Another challenge we encountered with animal behavior was related to the videofluoroscopic swallow studies. Use of the barium cookie allowed the rats pick up the cookie and move freely around the age, as opposed to the peanut butter barium mixture used in previous studies in our laboratory that was fixed to the front of the cage and ensured a standard distance from the C-arm across rats and swallows [114]. Ensuring equal distance might reduce some of the variability in magnification that could have contributed to variability in our findings. This would also allow us to control for animal size by using a spinal calibration during bolus measurements. This calibration method was not possible in this study because of variance in distance from the C-arm due to the freely moving rats.
Second, we did not quantify contractile properties of the tongue, including fatigue indices [91], and thus we cannot draw conclusions about differences between animal behavior (maximal voluntary press) and contractile properties derived through neuromuscular stimulation of the tongue muscles. This limitation prevents us from differentiating voluntary pressing as a learned behavior and muscle capacity. Third, we examined muscle biology in only one of the extrinsic muscles of the tongue—the genioglossus. Given that the other extrinsic and intrinsic tongue muscles have different activation patterns during tongue protrusion [89, 90], it is possible that there are differential effects of tongue exercise on mitochondrial structure and function in the retrusive muscles that we did not examine in this study. Future studies should explore exercise effects on these retrusive muscles as they work together with the GG to perform swallowing specific tasks [115, 116]. Also, we only examined muscle fiber type and fiber size changes at the midbelly of the GG muscle. Regional differences in this muscle do exist [74], therefore different findings may be found in the anterior or posterior regions. And finally, we did not measure O2 consumption from live tissue as a measure of mitochondrial function, which is the gold standard assay for determining health and function of regional mitochondria [110].
Future Directions
Given the limitations and scope of this study, much more work can be done within this animal model to further support the translation of these dosing characteristics to human participants. As discussed, accessing these muscles in the head and neck is not possible in healthy elders. Future studies using this model can investigate other dose components (intensity, repetitions, duration) and the effects on tongue muscle biology and physiology with the goal of optimizing dose effects and form better clinical recommendations. Future work should also explore other muscle characteristics that were not explored in this study, such as stimulated tongue force generative capacity vs voluntary tongue force generation and induced fatigue.
To clinically translate these findings, it will be necessary to conduct studies of varied exercise dose in human participants. Ideally, all components of exercise dose would be compared and tested and applied to development of a consensus on an optimal dose. Based on what we discovered in this work, it is likely that greater frequency of tongue exercise will produce larger changes in tongue force. However, our findings of change in force in 1 and 3 days/week of exercise are encouraging in that this limited dose resulted in a large percent change from baseline (51% change). Given that tongue force during swallowing is a submaximal task [117], determining the minimal change in tongue force necessary for improvement of swallowing will be critical for development of clinical guidelines. Another factor we must consider in determining appropriate dosing in human studies will be exercise adherence. Poor adherence to dysphagia treatments has been established [118]. This is a concern because of the strong relationship between adherence and achieving prescribed exercise dose. Researchers must consider feasibility of clinical programs for people with dysphagia when determining clinical doses for tongue exercise to maximize patient adherence.
Conclusion
This is the first study of resistance and endurance exercise in the cranial muscle system to examine related effects on muscle biology, and mitochondrial content and function. We conclude that increasing days/week of tongue exercise in the rat leads to greater increases in tongue force as measured by our voluntary tongue press task. Our findings indicate that 1 and 3 day(s)/week of exercise increase tongue force above control levels, but 5 days/week of exercise results in the greatest force increase. These changes in tongue force were not related to changes in muscle fiber type composition, fiber size, PGC-1α expression, or citrate synthase activity in the genioglossus muscle. Further, swallowing features (speed, bolus area, bolus size) showed high variability at baseline making change in bolus characteristics difficult to interpret. Because we were unable to define biological mechanisms of increased tongue forces with increasing exercise dose, more work must be done to examine other muscle variables, as discussed here. The findings of this study provide scientific understanding necessary for future clinical studies geared toward refining treatment approaches and maximizing outcomes.
References
Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112(4):589–602.
Roy N, et al. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–65.
Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Washington, DC: US Census Bureau; 2014. p. 25–1140.
Robbins J, et al. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–9.
Cook I, et al. (1994) Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol Gastrointest Liver Physiol. 1994;266(6):972–7.
Martin BJ, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9(1):1–6.
Mcconnel F. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98(1):71–8.
Tamine K, et al. Age-related changes in tongue pressure during swallowing. J Dent Res. 2010;89(10):1097–101.
Nicosia MA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A. 2000;55(11):M634–M640640.
Maeda K, Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30(1):80–7.
Yoshida M, et al. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21(1):61–5.
Namasivayam AM, Steele CM, Keller H. The effect of tongue strength on meal consumption in long term care. Clin Nutr. 2016;35(5):1078–83.
Altman KW, Yu G-P, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–9.
Cabre M, et al. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
Cichero JA, Altman KW. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources, in Stepping stones to living well with dysphagia. Basel: Karger Publishers; 2012. p. 1–11.
Eslick GD, Talley N. Dysphagia: epidemiology, risk factors and impact on quality of life–a population-based study. Aliment Pharmacol Ther. 2008;27(10):971–9.
Humbert IA, Robbins J. Dysphagia in the elderly. Phys Med Rehabil Clin N Am. 2008;19(4):853–66.
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s.
Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci. 1988;84(2):275–94.
Evans WJ, Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A. 1995;50(Special Issue):11–6.
Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81(11):1810–6.
Alnaqeeb M, Goldspink G. Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat. 1987;153:31.
Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–42.
Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol. 2002;27(1):19–411.
Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854(1):92–101.
Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128(2):351S–5S.
Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–27.
Frontera WR, Zayas AR, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys Med Rehabil Clin. 2012;23(1):201–7.
Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45(5):397–458.
Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol. 1997;22(4):307–27.
Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Technol. 2000;50(6):500–9.
Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223.
Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012. https://doi.org/10.1155/2012/194821.
Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci. 1996;93(26):15364–9.
Miquel J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch Gerontol Geriatr. 1991;12(2–3):99–117.
Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–8.
Boffoli D, et al. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226(1):73–82.
Carter HN, Chen CC, Hood DA. Mitochondria, muscle health, and exercise with advancing age. Physiology. 2015;30(3):208–23.
Adhihetty PJ, et al. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88(1):99–107.
Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–51.
Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24.
Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90.
Tipton CM, Medicine ACOS. ACSM's advanced exercise physiology. Philadelphia: Lippincott Williams & Wilkins; 2006.
Derbré F, et al. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age. 2012;34(3):669–79.
Gibala MJ, et al. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106(3):929–34.
Tanaka K, et al. Preventive effects of electrical stimulation on inflammation-induced muscle mitochondrial dysfunction. Acta Histochem. 2016;118(5):464–70.
Kawai N, et al. Adaptation of rat jaw muscle fibers in postnatal development with a different food consistency: an immunohistochemical and electromyographic study. J Anat. 2010;216(6):717–23.
Wright DC, et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282(1):194–9.
Brealey D, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. The Lancet. 2002;360(9328):219–23.
Shepherd D, Garland P. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969;114(3):597–610.
Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90(3):1137–57.
Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15(1):97–117.
Srere P. [1] Citrate synthase: [EC 4.1. 3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 1969;13:3–11.
Robbins J, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–8.
Lazarus C. Tongue strength and exercise in healthy individuals and in head and neck cancer patients. Seminars in speech and language. New York: Thieme Medical Publishers; 2006.
Oh JC. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia. 2015;30(3):315–20.
Park J-S, Kim H-J, Oh D-H. Effect of tongue strength training using the Iowa oral performance instrument in stroke patients with dysphagia. J Phys Ther Sci. 2015;27(12):3631–4.
Robbins J, et al. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53(9):1483–9.
Rogus-Pulia N, et al. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J Am Geriatr Soc. 2016;64(2):417–24.
Robbins J, et al. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A. 1995;50(5):M257–M262262.
Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–65.
Langmore SE, Pisegna JM. Efficacy of exercises to rehabilitate dysphagia: a critique of the literature. Int J Speech Lang Pathol. 2015;17(3):222–9.
Ferguson B. ACSM’s guidelines for exercise testing and prescription 9th Ed. J Can Chiropractic Assoc. 2014;58(3):328.
Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3:735.
Krisciunas GP, et al. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia. 2012;27(4):538–49.
Virani A, et al. Effects of 2 different swallowing exercise regimens during organ-preservation therapies for head and neck cancers on swallowing function. Head Neck. 2015;37(2):162–70.
McKenna VS, et al. A systematic review of isometric lingual strength-training programs in adults with and without dysphagia. Am J Speech Lang Pathol. 2017;26(2):524–39.
Peterson MD, Rhea MR, Alvar BA. Applications of the dose-response for muscular strength development: areview of meta-analytic efficacy and reliability for designing training prescription. J Strength Cond Res. 2005;19(4):950–8.
Connor NP, et al. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–44.
Connor NP, et al. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51(4):818–27.
Kletzien H, et al. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol. 2013;114(4):472–81.
Schaser AJ, Ciucci MR, Connor NP. Cross-activation and detraining effects of tongue exercise in aged rats. Behav Brain Res. 2016;297:285–96.
Krekeler BN, Connor NP. Age-related changes in mastication are not improved by tongue exercise in a rat model. Laryngoscope. 2017;127(1):E29–e34.
Schaser AJ, et al. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2011;26(3):256–63.
Russell JA, et al. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28(1):95–104.
Lenth RV (2006) Java applets for power and sample size [computer software]. 9.
Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. J Am Assoc Lab Anim Sci. 2000;39(6):9–17.
Cullins MJ, Connor NP. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve. 2017;56:E119–E12525.
Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE. 2012;7(4):e35273.
Schiaffino S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10(3):197–205.
Lucas CA, Kang LH, Hoh JF. Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun. 2000;272(1):303–8.
Eddinger TJ, Moss RL, Cassens RG. Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J Histochem Cytochem. 1985;33(10):1033–41.
Smith LR, Barton ER. SMASH-semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skeletal muscle. 2014;4(1):1.
Li R, Shen Y. An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013;92(13):747–51.
Eaton SL, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE. 2013;8(8):e72457.
Antharavally BS, et al. A high-affinity reversible protein stain for Western blots. Anal Biochem. 2004;329(2):276–80.
McLester JR, Bishop E, Guilliams M. Comparison of 1 day and 3 days per week of equal-volume resistance training in experienced subjects. J Strength Cond Res. 2000;14(3):273–81.
Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2010;42(5):902–14.
Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97(1):933–6.
Palmer PM, et al. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51(4):828–35.
Ota F, Connor NP, Konopacki R. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol. 2005;114(10):799–803.
Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52(2):438–44.
Bole C 2nd, Lessler MA. Electromyography of the genioglossus muscles in man. J Appl Physiol. 1966;21(6):1695–8.
Cunningham DP, Basmajian JV. Electromyography of genioglossus and geniohyoid muscles during deglutition. Anat Rec. 1969;165(3):401–9.
Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–88.
Spiering BA, et al. Resistance exercise biology. Sports Med. 2008;38(7):527–40.
Häkkinen K, Alen M, Komi P. Changes in isometric force-and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol Scand. 1985;125(4):573–85.
Cartee GD, et al. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23(6):1034–47.
Wang L, et al. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J Appl Physiol. 2011;111(5):1335–444.
Lysenko E, et al. Effect of combined aerobic and strength exercises on the regulation of mitochondrial biogenesis and protein synthesis and degradation in human skeletal muscle. Hum Physiol. 2016;42(6):634–44.
Granata C, et al. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016;30(10):3413–23.
Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. 2016;101(1):17–22.
Schwarz NA, et al. Effect of resistance exercise intensity on the expression of PGC-1α isoforms and the anabolic and catabolic signaling mediators, IGF-1 and myostatin, in human skeletal muscle. Appl Physiol Nutr Metab. 2016;41(8):856–63.
Coffey VG, et al. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab. 2006;290(5):E849–E855855.
Alvehus M, et al. Metabolic adaptations in skeletal muscle, adipose tissue, and whole-body oxidative capacity in response to resistance training. Eur J Appl Physiol. 2014;114(7):1463–71.
Salvadego D, et al. Skeletal muscle oxidative function in vivo and ex vivo in athletes with marked hypertrophy from resistance training. J Appl Physiol. 2013;114(11):1527–35.
Tesch P, Komi P, Häkkinen K. Enzymatic adaptations consequent to long-term strength training. Int J Sports Med. 1987;8(S1):S66–S69.
Wang N, et al. Muscle fiber types of women after resistance training—quantitative ultrastructure and enzyme activity. Pflügers Arch. 1993;424(5–6):494–502.
Porter C, et al. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2015;47(9):1922.
Horan M, Pichaud N, Ballard JW. Quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A. 2012;67(10):1022–35.
Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–5.
Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15(2):161–7.
Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci. 1998;95(3):861–8.
Kletzien H, Cullins MJ, Connor NP. Age-related alterations in swallowing biomechanics. Exp Gerontol. 2019;118:45–50.
Amri M, Lamkaden M, Car A. Activity of extrinsic tongue muscle during swallowing in sheep. Brain Res. 1989;503(1):141–3.
Napadow VJ, et al. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol Gastroint Liver Physiol. 1999;277(3):G695–G701.
Robbins J, et al. Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia. 2016;31(1):90–6.
Krekeler BN, et al. Patient adherence to dysphagia recommendations: a systematic review. Dysphagia. 2018;33(2):173–84.
Acknowledgements
This work was supported by NIH Grants 1F31AG059351-01, R01DC018071, R01DC008149, R01DC014358, R37CA225608. This manuscript was submitted in partial fulfillment of the requirements for the doctoral dissertation of the first author, BNK. The authors would like to acknowledge the help of Dr. John Russell during animal tissue harvest, Jared Cullen for his help in coordination of animal training, Drs. Heidi Kletzien. Tiffany Glass, and Miranda Cullins for their encouragement and support in the learning of these assays. The first author would like to thank and recognize her dissertation committee members for their valuable contributions to her training and feedback on this manuscript: Drs. Nicole Rogus-Pulia, Michelle R Ciucci, Gary Diffee, Catriona Steele, and Timothy McCulloch; and Dr. Glen Leverson for his statistical mentorship throughout her dissertation work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krekeler, B.N., Weycker, J.M. & Connor, N.P. Effects of Tongue Exercise Frequency on Tongue Muscle Biology and Swallowing Physiology in a Rat Model. Dysphagia 35, 918–934 (2020). https://doi.org/10.1007/s00455-020-10105-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10105-2