Abstract
It has been demonstrated that the insular cortex plays an important role in the swallowing mechanism. This case report describes a patient with bilateral insular cortex lesions and dysphagia secondary to viral meningitis. Recent evaluations of the insula’s role in the swallowing mechanism are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The patient is a 33-year-old Caucasian woman referred to the Epilepsy Monitoring Unit (EMU) for evaluation of episodic slurred speech and dysphagia, possibly epileptic in nature. Twelve weeks prior to admission she presented with fever, bilateral facial and neck numbness, generalized lethargy, and altered level of consciousness. She was admitted to a local hospital. She was described as having very hypophonic nasal speech and had significant difficulty protecting her airway. Aspiration occurred shortly after admission and the patient required intubation. A lumbar puncture was performed and the cerebral spinal fluid (CSF) profile showed 226 white blood cells (WBC) (75% lymphocytes) 4 red blood cells (RBC), glucose of 100 mg/dl, and protein of 58 g/dl. A cerebral magnetic resonance imaging examination (MRI) initially was unremarkable, but subsequently two MRIs demonstrated abnormal T2 prolongation in amygdala, anterior temporal lobe, and insular cortical and subcortical regions. The abnormal signal had a bilateral symmetrical distribution. A diagnosis of viral encephalitis was tentatively established. The CSF polymerase chain reaction (PCR) for herpes simplex virus (HSV) was negative. Several days later she had improved and was easily weaned off the ventilator and extubated. She had an inpatient course of gradual improvement. At the time of admission to our EMU, the patient had markedly improved but continued to complain of episodic slurred speech, associated with unusual sensations in her throat and difficulty swallowing. The unusual sensation in her throat was followed by episodes of repetitive swallowing, typically lasting one to three minutes. She was referred for monitoring to determine if these episodes represented partial seizures.
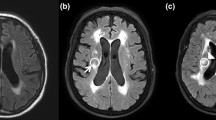
The patient’s baseline electroencephalogram (EEG) was normal. During monitoring, the patient experienced multiple events of abnormal sensations in her throat with mild slurred speech. These events were associated with repeated swallowing of saliva. There was no background change in the patient’s EEG during this time period. During her monitored hospitalization, the patient also underwent repeat MRI, lumbar puncture, and videofluoroscopic swallowing examination (VFSE). Her lumbar puncture at that time was normal. MRI, three months after the onset of symptoms, continued to show T2 prolongation involving bilateral amygadala, anterior temporal lobes, and insular cortex (Fig. 1). The results of the speech and swallow evaluations are shown in Table 1.
Neurologic Examination
The patient had a full neurological examination at the time of admission. Her higher cortical function was completely normal, including language and memory. On cranial nerve testing, the patient displayed a bilaterally reduced gag reflex but was otherwise normal. The patient’s tongue protruded midline and there was no facial asymmetry. No buccofacial apraxia was seen. Motor strength testing, reflexes, and cerebellar function were normal. She had no frontal lobe release signs and no pathological reflexes.
Speech and Language Assessment
The patient complained of having to exert extra effort to swallow, recent onset of reflux symptoms, occasional regurgitation, difficulty chewing, and pain from the jaw to the temples, which worsened with chewing. The results of the formal speech evaluation are listed in Table 1. Both clinical and videofluoroscopic swallowing examinations were completed. The VFSE comprised two 5-ml thin liquid boluses, two 5-ml honey-thick boluses, a self-administered gulp, two semisolid boluses, and a quarter of a sugar cookie in the lateral view and two 5-ml thin liquid boluses in the anterior–posterior view. Testing revealed a moderate risk for aspiration because of delayed initiation of the swallow, obvious on both clinical and VFSE. Aspiration of a small portion of one honey-thick liquid bolus and substantial residual of all bolus types in both valleculae and pyriform sinuses was seen on the VFSE. The patient was observed to have a dysarthric slow rate of speech characterized by a breathy dysphonia and hypernasality. Based on these results, the speech-language pathologist determined the diagnosis to be a motor speech disorder and swallowing disorder. The patient was instructed to reduce bite size, eat moist foods, and to use frequent liquid washes to compensate for these deficits.
Discussion
The patient described in this case presented with bilateral lesions of the insular cortex. The severity of deficits at presentation would indicate that bilateral lesions result in profound dysfunction of the swallowing mechanism.
The insula is the area of cortex that covers the medial wall of the sylvian cistern. The insular cortex is included as part of the paralimbic cortex and has primarily been considered to play a role in the visceral sensory functions of taste and olfactory sensations as well as gastrointestinal and respiratory motor functions. Areas of connectivity include the premotor cortex, the nucleus tractus solitarius (NTS), the thalamus, olfactory and gustatory areas, and limbic and autonomic areas. The insular cortex, in addition to the above areas, has also been shown to have connections to preganglionic parasympathetic motor neurons.
Early insight into insular function was obtained using direct cortical stimulation. Characteristic visceral sensory phenomena described by patients following stimulation involved an unusual feeling in the abdomen occasionally associated with nausea and pain which was often described as simply an abnormal abdominal “sensation” [1]. There were also occasions of associated chewing, swallowing, salivation, and inhibition of respiration with direct stimulation [1].
The insular cortex’s role in speech articulation and swallowing mechanisms has been a more recent area of interest. Daniels et al. [2,3] published two studies addressing the role of the insular cortex in dysphagia. The initial study presented 16 patients, who had unilateral stroke and dysphagia, with documented lesions of the insula in 11 of the 16 patients [2]. They then reported on four additional patients with discrete unilateral insular lesions [3]. Three of the four patients demonstrated dysphagia by videofluroscopy [3]. In each of these three patients, the lesion site was the anterior insula as opposed to the one patient without dysphagia and a posterior insular lesion [3]. These results suggested the anterior insula as a more specific site of swallowing function. This seems to be reinforced by experience with direct stimulation on the anterior insula resulting in sensory responses in the mouth, throat and tongue [1].
The insula has also been implicated in the motor planning of speech [4]. Through computer reconstructions of lesions demonstrated on computed tomography (CT) and MRI, 25 stroke patients with a disorder in motor planning were each found to have a common insular lesion [4]. Although speech apraxia was not present in our case, it is likely that the insula plays a similar role in the motor planning of swallowing.
The cortical topography of human swallowing has also been demonstrated to have a bilateral representation [5]. Through cortical stimulation, it has been shown that individual muscle groups involved in swallowing are bilaterally represented on the motor and premotor cortex [5]. Given this finding, our patient with bilateral lesions would be expected to have a severe clinical course.
There are relatively few reported cases of bilateral insular cortex lesions, but a single published report demonstrated bilateral insular cortex infarctions that resulted in severe buccofacial apraxia and mutism [6]. On initial examination, that patient was found to have severe swallowing difficulties. The severity of dysphagia seen in that patient mirrors that experienced in the subject of this case report.
The exact mechanism by which the insular lesion results in dysphagia appears to involve multiple pathways. A primary component is related to the visceral input required to complete the swallowing process. The likely pathway crucial to this dysfunction would be between the insula and the NTS. A visceral sensory deficit, in combination with a disruption in the connections to the primary gustatory cortex and paravicellar division of the ventroposteromedial nucleus of the thalamus, would suggest a multifactorial cause of dysphagia from an insular lesion. Impairment of the connectivity between the insula and primary and supplementary motor cortices likely results in dysfunction of the motor planning of the swallowing mechanism.
Our patient demonstrates the importance of the insula in the mechanics of swallowing. The areas of involvement in this patient included both anterior and posterior insular cortical and subcortical regions resulting in disruption of the various connections described above. To our knowledge this case represents only the second case to document the severity of deficits that can occur with bilateral insular cortex lesions.
References
P Wilder M Falk (1995) ArticleTitleThe insula: further observations on its function. Brain 78 445–469
S Daniels A Foundas G Iglesia M Sullivan (1996) ArticleTitleLesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovas Dis 6 30–34
S Daniels A Foundas (1997) ArticleTitleThe role of the insular cortex in dysphagia. Dysphagia 12 146–156 Occurrence Handle1:STN:280:ByiA3szpvFE%3D Occurrence Handle9190100
N Dronkers (1996) ArticleTitleA new brain region for coordinating speech articulation. Nature 384 159–161 Occurrence Handle1:CAS:528:DyaK28XmvFGhtr4%3D Occurrence Handle8906789
S Hamdy Q Aziz J Rothwell K Singh J Barlow D Hughes R Tallis D Thompson (1996) ArticleTitleThe cortical topography of human swallowing musculature in health and disease. Nat Med 2 1217–1224 Occurrence Handle1:CAS:528:DyaK28Xms1Wqt7g%3D Occurrence Handle8898748
M Habib G Daquin L Milandre M Royere M Rey A Lanteri G Salamon R Khalil (1995) ArticleTitleMutism and auditory agnosia due to bilateral insular damage—role of insula in human communication. Neuropsychologia 33 327–339 Occurrence Handle1:STN:280:ByqA3M3itFY%3D Occurrence Handle7791999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stickler, D., Gilmore, R., Rosenbek, J.C. et al. Dysphagia with Bilateral Lesions of the Insular Cortex . Dysphagia 18, 179–181 (2003). https://doi.org/10.1007/s00455-002-0103-2
Issue Date:
DOI: https://doi.org/10.1007/s00455-002-0103-2