Abstract
A new aerobic denitrifying bacterium ZYL was isolated from domestic wastewater sludge and identified as Acinetobacter haemolyticus (similarity 99%) by the 16S rDNA sequencing analysis. The strain could use nitrate, nitrite and ammonium as the sole N-source for growth with a final product of N2, demonstrating its good abilities for aerobic denitrification and heterotrophic nitrification. Single-factor experiment results showed that the effective removal of nitrate by strain ZYL occurred with carbon source sodium succinate, C/N 16–24, pH 5–9, temperature 20–40 °C, DO ≥ 4.84 mg/L. Ammonium was preferentially used by strain ZYL with nitrate and ammonium as the mixed nitrogen sources. According to nitrogen utilization, nitrogen balance analysis, enzyme assay and denitrifying gene amplification, nitrate was assimilated directly by the isolate for cell synthesis and also converted into N2 through aerobic denitrification. All these make strain ZYL an ideal choice for treating nitrogen-containing wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the over-consumption of nitrogen fertilizers and the intensive development of industry activities, nitrogen pollution that can cause eutrophication of water bodies and affect human health has become more and more serious [1, 2]. Thus, the nitrogen removal from wastewater has become one of the most important problems in water pollution control. Biological methods are widely used for nitrogen removal due to their merits such as no secondary pollution, high-efficiency and cost benefit [3]. Traditionally, nitrogen removal process involves aerobic nitrification by autotrophic nitrifiers and anoxic denitrification by anoxic denitrifiers [4]. Obviously, the key to high-efficiency nitrogen removal is the successive exposure of wastewater under aerobic and anoxic conditions, but this is difficult to assure a strict anoxic environment by completely removing dissolved oxygen (DO) from wastewater. Therefore, the denitrifying performance of most denitrifiers is inhibited by the insufficient anoxic condition, leading to the decrease in removal efficiency of nitrogen.
Aerobic denitrification is first reported by isolated Paracoccus denitrificans, which is able to reduce nitrate or nitrite to N2 under aerobic culture condition [5]. This property can overcome the requirement of strictly anoxic environmental condition in the traditional process and achieve simultaneous nitrification and denitrification (SND) in the same reactor under aerobic condition. Subsequently, many bacteria with aerobic denitrifying ability have been isolated and studied one after another [6,7,8,9]. Taking the genus of Acinetobacter as an example, Zhao et al. reported that Acinetobacter calcoaceticus HNR demonstrates aerobic denitrification through hydroxylamine, rather than nitrate and nitrite [10]. Ren et al. reported that nitrate and nitrite can be used as the sole N-source for growth by Acinetobacter junii YB and provided as an energy source for its aerobic denitrification [11]. Yang et al. found that Acinetobacter sp. JR1 indicates excellent nitrate removal abilities under acidic condition [9]. All these investigations indicate that these aerobic denitrifying bacteria, even belonging to the same genus, show different aerobic denitrifying characteristics. Thus, it is of great importance to further study the aerobic denitrifying characteristics of more aerobic denitrifying strains, which is benefit to their practical application in wastewater treatment.
In this study, a novel strain ZYL with excellent denitrifying performance was successfully isolated and identified as Acinetobacter haemolyticus. Previous researches about Acinetobacter haemolyticus mainly focus on the bio-removal of Cr6+ [12], diesel [13] and alkane [14]. However, there are few studies on the genus Acinetobacter haemolyticus to remove nitrogen. Thus, the single-factor experiment was carried out to optimize the conditions for aerobic denitrification of strain ZYL. Second, we also systematically analyze the utilization of different nitrogen sources of strain ZYL to further understand its heterotrophic nitrification and aerobic denitrification ability. Finally, the nitrogen removal pathway of strain ZYL was preliminarily determined by gas detection, nitrogen balance analysis, key enzyme assay and denitrifying gene amplification. The in-depth understanding of the nitrogen removal performance of the isolate will be conducive to its application in treating actual wastewater containing nitrogen.
Materials and methods
Media
The LB medium used for bacteria enrichment included the following ingredients (per liter): 5 g of yeast extract, 10 g of NaCl, 10 g of tryptone, pH 7.0.
The denitrification medium (DM) used for bacteria isolation and aerobic denitrification test contains the following ingredients (per liter): 0.72 g of KNO3 (or 0.49 g of NaNO2), 5.79 g of sodium succinate, 0.75 g of K2HPO4, 0.25 g of NaH2PO4, 0.12 g of NaCl, 0.01 g of MgSO4·7H2O, 0.01 g of MnSO4·H2O, 0.01 g of FeSO4·7H2O, pH 7.0.
The nitrification medium (NM) used for heterotrophic nitrification test contains the following ingredients (per liter): 0.48 g of (NH4)2SO4, 5.79 g of sodium succinate, 0.75 g of K2HPO4, 0.25 g of NaH2PO4, 0.12 g of NaCl, 0.01 g of MgSO4·7H2O, 0.01 g of MnSO4·H2O, 0.01 g of FeSO4·7H2O, pH 7.0.
The simultaneous nitrification and denitrification mixed medium (SNDM) was prepared from the following components: 0.36 g of KNO3, 0.24 g of (NH4)2SO4, 5.79 g of sodium succinate, 0.75 g of K2HPO4, 0.25 g of NaH2PO4, 0.12 g of NaCl, 0.01 g of MgSO4·7H2O, 0.01 g of MnSO4·H2O, 0.01 g of FeSO4·7H2O, pH 7.0.
All chemical reagents were of analytical purity and used directly without purification after procurement. Solid media were prepared from above media with the addition of 1.8% agar, and all media were autoclaved at 121 °C for 20 min before use.
Isolation and identification of strain ZYL
The source of the bacterium was activated sludge which was collected from the secondary settling tank of a sewage treatment plant (Taiyuan, china). The fresh sludge (2 mL) was transferred to a 250 mL Erlenmeyer flask containing 200 mL of fresh LB medium, and incubated at 120 rpm and 30 °C to obtain homogeneous bacterial suspension. Subsequently, this bacterial suspension (1 mL) was pipetted into another sterile Erlenmeyer flask containing 100 mL of fresh DM for selective cultivation of denitrifying bacteria (repeated this procedure three times). After tenfold serial dilution, the suspension (0.1 mL) was spread on DM agar plates and cultured at 30 °C to obtain bacterial colonies. And then, the colonies with different appearances were selected and streaked on the fresh agar DM plates. Several purified colonies were obtained and tested their aerobic denitrifying abilities. Ultimately, a strain with excellent denitrifying ability was obtained, and stored at − 80 °C in form of bacteria suspension with glycerol solution (30% v:v).
The genomic DNA of strain ZYL was used as the template for the 16S rDNA gene amplification and its primers were F27 (5′-AGAGTTTGATCMTGGCTCAG-3′) and R1492 (5′-TTGGYTACCTTGTTACGACT-3′). Subsequently, the 16S rDNA PCR products were sequenced by Takara Biotechnology (Dalian, China) Co., Ltd, and its sequences, which were submitted to GenBank to obtain the accession number, were compared with those of other related microorganisms by BLAST. Using the neighbor-joining method with 1000 bootstrap replicates and the maximum composite likelihood model, a phylogenyetic tree was finally built in MEGA 5.1 software.
Effects of influencing factors on nitrate removal
Single-factor experiment was carried out to study the influencing factors such as carbon source, C/N ratio, pH, temperature, dissolved oxygen and nitrate concentration on the nitrate removal under aerobic condition. For optimizing its carbon source, strain ZYL was cultured in denitrification medium at 30 °C and 120 rpm with the following carbon sources: sodium succinate, fumaric acid, sodium acetate, sodium pyruvate and glucose. In C/N ratio experiments, the C/N ratios were set at 4, 8, 12, 16, 20 and 24 with a constant nitrate concentration of 100 mg/L, at 30 °C and 120 rpm. For the pH experiment, the initial pH was set to 4, 5, 6, 7, 8, 9 and 10, respectively, at 30 °C and 120 rpm. In the temperature experiment, the temperature was set at 10, 20, 30 and 40 °C, respectively, at pH 7 and 120 rpm. For the dissolved oxygen (DO) experiment, the shaking speed was set to 0 (DO ≈ 1.43 mg/L), 40 (DO ≈ 2.58 mg/L), 80 (DO ≈ 3.46 mg/L), 120 (DO ≈ 4.84 mg/L), 160 (DO ≈ 5.52 mg/L) and 200 rpm (DO ≈ 6.22 mg/L) in the DM, respectively, at pH 7 and 30 °C. For the initial nitrate concentration experiments, its corresponding concentrations were adjusted to 50, 100, 200, 300, 500, 800 and 1000 mg/L, at pH 7, 30 °C and 120 rpm. All the experiments were operated with 1% (v/v) inocula (OD600 ≈ 1.01) into 250 mL bottles containing 100 mL of aseptic media, and the corresponding non-inoculated media were used as the controls. Moreover, all media were autoclaved at 121 °C for 20 min except the medium used glucose as the sole carbon source was autoclaved at 110 °C for 15 min. The cultures were sampled periodically to determine OD600 and pH, and their supernatants after centrifugation were used to measure the levels of TN, COD, \({\text{NO}}_{{3}}^{ - }\)–N, \({\text{NO}}_{{2}}^{ - }\)–N and \({\text{NH}}_{{4}}^{ + }\)–N.
Nitrogen utilization by strain ZYL
For the aerobic denitrifying ability, nitrate and nitrite were used as the sole N-sources with an initial concentration of 100 mg/L, respectively. At the same time, the heterotrophic nitrification ability of the strain was studied using ammonium with an initial concentration of 100 mg/L as the sole N-source. For the simultaneous nitrification and denitrification ability, both nitrate (50 mg/L) and ammonium (50 mg/L) were used as the mixed nitrogen sources. All the experiments were operated with 1% (v/v) inocula (OD600 ≈ 1.01) into 250 mL bottles containing 100 mL of aseptic media, and the corresponding non-inoculated media were used as the controls. The cultures were sampled periodically to determine OD600 and pH, and their supernatants after centrifugation were used to measure the levels of TN, COD, \({\text{NO}}_{{3}}^{ - }\)–N, \({\text{NO}}_{{2}}^{ - }\)–N and \({\text{NH}}_{{4}}^{ + }\)–N.
Nitrate removal pathway analysis
The experiments such as gas detection, nitrogen balance analysis, key enzyme assay and denitrifying gene amplification were performed to determine the nitrate removal of strain ZYL. For the gas detection and nitrogen balance experiment, 1 mL of activated bacterial suspension was inoculated into a 500 mL sealed bottle containing 50 mL of sterilized DM medium, and then the bottles were filled with a volume ratio of high purity oxygen (99.99%) and high purity argon (99.99%) of 1:1. These bottles were tightly sealed and cultured at 30 °C and 120 rpm for 36 h, and the bottles without inoculated culture were used as the controls. Gas samples of 1 mL were collected at 0 and 36 h, and then O2 and N2 were analyzed by gas chromatograph with a thermal conductivity detector. Meanwhile, the nitrogen balance experiment was performed in the nitrate biodegradation process. The culture samples were collected at 0 and 36 h, respectively, and their supernatants after centrifugation were used to measure the levels of TN, \({\text{NO}}_{{3}}^{ - }\)–N, \({\text{NO}}_{{2}}^{ - }\)–N, \({\text{NH}}_{{4}}^{ + }\)–N and intracellular nitrogen content.
For enzyme assay, the specific activity (U/mg) of the nitrate reductase (NR) and the nitrite reductase (NIR) were determined by the methods described in our previous studies [15], and the samples was prepared using the following process: by centrifuging at 10,000 rpm at 4 °C for 10 min, strain ZYL was harvested at the 6, 12, 18, 24, 36 and 48 h at 30 °C and 120 rpm in DM, and these bacterial sediment were then resuspended in 10 mM phosphate buffer (pH 7.4). After lysing the above bacterial suspension via ultrasonication treatment, the cell-free extracts were obtained by centrifuging at 12,000 rpm at 4 °C for 20 min and used to determine the enzyme activity of NR and NIR.
For the denitrifying gene test, the genomic DNA of strain ZYL was extracted according to the bacterial DNA extraction kit (Sangon, Shanghai, China), which was served as the DNA template for the amplification of nitrate reductase gene (napA) and nitrite reductase gene (nirS). The amplification primers of napA were F (5′-CGCAGATCAATTCCAAGCGT-3′) and R (5′-TAAGCCACCCACTTCACGAC-3′). The amplification primers of nirS were F (5′-CAGACAGCGCAACGATTTGT-3′) and R (5′-CATTCCACCATTACCACACACA-3′). These PCR products were sequenced directly by Sangon Biotechnology Co., Ltd (Shanghai, China), and the gene sequences were submitted to GenBank to obtain the accession number, and then analyzed by the BLAST tool.
Analytical methods
Cell densities were determined by monitoring the OD600 using as pectrophotometer (Spectrum instruments, Shanghai). pH was determined with a pH meter (PB-10, Sartorius, Germany), and dissolved oxygen was determined with a DO meter (HQ30D, HACH, USA). COD was determined by the potassium dichromate method using a COD analyzer (DR 1010, HACH, USA). TN and intracellular nitrogen was determined by alkaline potassium persulfate digestion-UV spectrophotometric method. \({\text{NO}}_{{3}}^{ - }\)–N was measured by phenol disulfonic acid method at 410 nm, \({\text{NO}}_{{2}}^{ - }\)–N was determined by the method of N-(1-naphthalene)-diaminoethane spectrophotometer at 540 nm, and \({\text{NH}}_{{4}}^{ + }\)–N was determined by the method of Nessler’s reagent photometry at 420 nm.
Intracellular nitrogen concentration was calculated by the formula TN0 − TN1. TN0 is the TN content of non-centrifuged inoculated medium, and TN1 is the TN content of inoculated medium following centrifugation (8000 rpm for 10 min) [16]. The corresponding removal efficiency and removal rate were calculated by the formula (C0 − Ct)/C0 × 100% and (C0 − Ct)/t, respectively. C0 is the initial concentration of nitrogen (TN, \({\text{NO}}_{{3}}^{ - }\)–N, \({\text{NO}}_{{2}}^{ - }\)–N, \({\text{NH}}_{{4}}^{ + }\)–N) and COD, Ct represents the concentration of nitrogen (TN, \({\text{NO}}_{{3}}^{ - }\)–N, \({\text{NO}}_{{2}}^{ - }\)–N, \({\text{NH}}_{{4}}^{ + }\)–N) and COD at t·h, and t is the time of strain ZYL treatment. All the experiments were carried out in triplicate, and statistical analysis was performed using Excel and Origin 9.0.
Results and discussion
The identification of strain ZYL
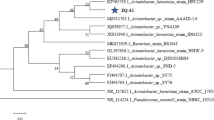
A strain with good nitrate removal ability under aerobic conditions was isolated and named ZYL. Strain ZYL is a Gram-negative bacterium appearing as short rods and existing individually. Its colony is rounded, creamy-white, smooth surface and with a regular edge in the agar plate. Nearly full-length 16S rDNA gene (1452 bp) of strain ZYL was sequenced, and its sequences were deposited in GenBank with an accession number of MH636368. The BLAST results assign strain ZYL to the genus Acinetobacter haemolyticus with the highest similarity of 99%. According to the 16S rDNA gene sequence of isolates and other related strains, a phylogenetic tree was built. As shown in Fig. 1, the result further confirms that the identification of strain ZYL as Acinetobacter haemolyticus. Thus, the present strain ZYL belongs to Acinetobacter haemolyticus species. Previous researches have reported that the genus Acinetobacter includes many strains showing excellent denitrification abilities under aerobic culture condition, such as Acinetobacter junii YB [11], Acinetobacter sp. JR1 [9] and Acinetobacter harbinensis HITLi7T [7], and these different strains have different characteristics for nitrogen removal. Therefore, the current study mainly focuses on investigating the aerobic denitrifying capacity of strain ZYL.
Effects of carbon source, C/N ratio, temperature, pH, dissolved oxygen and initial nitrate concentration on nitrate removal under aerobic culture condition
Carbon source
As we all know, the carbon source is a significant factor that influences the growth and aerobic denitrification of microbes, because it usually provides energy and electron donors for heterotrophic microorganisms [11]. Thus, effects of different carbon sources on the cell growth and nitrate removal were studied at 120 rpm and 30 °C with a fixed C/N ratio of 14, and it can be concluded from Fig. 2a that the cell growth and nitrate removal are greatly affected by carbon source types (P < 0.05). For strain ZYL, sodium succinate seems to be the most suitable carbon source for its cell growth and nitrate removal among the five carbon sources used in this study, with evidence that the highest OD600 (1.12) and nitrate removal (86.16%) are obtained after 36 h (P < 0.05). However, minimal growth (OD600 ≈ 0.06) and the worst nitrate removal (1.82% at 48 h) of strain ZYL is observed when glucose is used as the sole carbon source, indicating that strain ZYL could not utilize glucose as the sole carbon source for growth. Obviously, nitrate could not be completely removed by strain ZYL even with sodium succinate as the sole carbon source. This may be due to the insufficient supply of carbons with evidence by the highest COD removal (98.16%) after 36 h (Fig. 2a). Moreover, the TN and nitrate removal also shows same variation trend, because there is almost no ammonium and nitrite accumulation during the nitrate removal process (data not shown in Fig. 2a). Considering the cell growth and aerobic denitrifying ability, sodium succinate is chosen as the carbon source in the next study.
C/N ratio
Figure 2b shows the influence of different C/N ratios on the growth and nitrate removal of strain ZYL. These results demonstrate that both the growth and nitrate removal show similar trend, and they both increase with the increment of C/N ratios in the range of 4–20, probably is because of the insufficient supply of carbon, which weakens microbial growth and electron donor for denitrifying [11, 17, 18]. At the C/N ratio of 20 and 24, 100% of nitrate is removed by strain ZYL. However, there is an obvious decrease in COD removal efficiency at C/N ratio of 24 within 48 h, which might be due to the depletion of nitrogen source, resulting in low COD degradation as the carbon supply is higher than its requirement. Moreover, TN removal is consistent with nitrate removal, and the removal efficiencies of TN are 41.62%, 56.81%, 78.54%, 91.60%, 98.45% and 96.63% at C/N ratios of 4, 8, 12, 16, 20 and 24, respectively. As a result, the relatively high C/N ratios are more beneficial to bacterial growth and nitrate degradation, and the C/N ratio of 20 might be the best condition for efficient nitrate removal by strain ZYL. Thus, strain ZYL might be suitable for treating some food industry wastewater which contains much higher carbons, leading to better growth for bacteria.
Initial pH
The effects of initial pH on the growth and nitrate degradation are depicted in Fig. 2c (P < 0.05). Almost no growth and nitrate degradation are observed at the initial pH of 4 and 10 within 48 h, which may be due to strong acidic or alkaline environment is harmful to the bacteria. However, strain ZYL grows well in the pH range of 5–9, and the maximum OD600 values are 0.95, 0.99, 1.19, 1.01 and 0.94 at pH 5, 6, 7, 8 and 9, respectively. Meanwhile, 69.58%, 96.74%, 100%, 98.18% and 80.28% of nitrate are degraded by ZYL within 36 h when the initial pH is 5, 6, 7, 8 and 9, respectively. After 48 h, nearly all of nitrate could be degraded, and the nitrate degradation efficiencies at pH 5, 6, 7, 8 and 9 are 99.99%, 99.65%, 100%, 97.66% and 99.08%, respectively. All these indicate that strain ZYL prefers a neutral or slightly alkaline condition, which is similar to other aerobic denitrifying bacteria [8, 19,20,21,22,23]. Thus, strain ZYL can grow well and degrade nitrate in the pH range of 5–9, and these pHs are consistent with the actual wastewater, benefiting for its practical applications.
Temperature
As illustrated in Fig. 2d, the growth and nitrate removal of strain ZYL are significantly influenced by temperature (P < 0.05). At 10 °C, the OD600 and nitrate degradation efficiency are only 0.14 and 22.33% at 48 h, respectively, probably because the growth-related genes would accordingly decrease under low temperature stress [24], and thus result in the decrease in the removal of nitrate. While the culture temperature rises from 20 to 30 °C, all of nitrate could be removed by strain ZYL at 48 h and 36 h, respectively, and this excellent nitrate removal at higher temperatures may be owing to the increased enzyme activities for denitrification. At the temperature increases up to 40 °C, the nitrate degradation decreases with the maximum removal efficiency of 85.63% at 48 h. Moreover, the accumulation of nitrite at 40 °C is much higher than that at other temperatures at 48 h. All these indicate that this temperature (40 °C) is too high for strain ZYL, probably further inhibiting the enzyme activity for denitrification, and thus resulting in the low nitrate removal and accumulation of nitrite. As a result, the optimal temperature for strain ZYL is 30 °C, which could be verified from the highest nitrate removal efficiencies (100%) and the fastest average degradation rate (2.80 mg/L/h) under this temperature. Thus, the resistance of strain ZYL to temperature is comparable to those of previously reported bacteria [15, 25, 26], benefiting for its application in a wide temperature range.
Dissolved oxygen
The effects of DO on the cell growth and nitrate removal were investigated and the rotational speed of the shaker was adjusted to control the concentration of DO in liquid media. As shown in Fig. 2e, the results indicate that the growth of microorganisms increases with the increase of rotation speed from 0 to 200 rpm (the corresponding concentration of DO is 1.43–6.22 mg/L), and the maximum OD600 values are 0.45, 0.48, 0.86, 1.19, 1.31 and 1.36, respectively. Moreover, the maximum removal efficiency of nitrate is significantly increased from 36.94% at 0 rpm (DO ≈ 1.43 mg/L) to 100% at 120 rpm (DO ≈ 4.84 mg/L). Low degradation efficiency of substrate may be attributed to insufficient dissolved oxygen [27]. However, with the further increase of rotational speed to 200 rpm (DO ≈ 6.22 mg/L), there is no obvious change in nitrate removal (P > 0.05). Increasing the rotational speed is beneficial to the increase of oxygen mass transfer coefficient, which significantly promotes cell growth and nitrate removal [16]. In view of nitrate removal efficiency and energy consumption, 120 rpm (DO ≈ 4.84 mg/L) is selected for the next study.
Initial nitrate concentration
Figure 2f illustrates the influence of initial nitrate concentration on nitrate biodegradation under aerobic conditions, and these results indicate that the amount of removed nitrate increases with the increment of the initial nitrate concentration. After 96 h of culture, the maximum amount of removed nitrate by strain ZYL is 52.78, 100.73, 197.01, 212.93, 256.26, 267.97 and 317.32 mg/L when the initial nitrate concentration is 52.78, 100.73, 201.87, 301.46, 503.66, 823.74 and 1033.74 mg/L, respectively. The highest removal efficiency of nitrate (100%) is achieved at 100 mg/L and then drops (P < 0.05). In contrast, it has been reported that the maximum removal of Rhodobacter sphaeroides ADZ101 with 144 h reaches 60.35, 95.85, 127.30, 150.40 and 151.34 mg/L when the initial nitrate concentrations are 85, 135, 190, 235 and 329 mg/L, respectively. Moreover, strain ADZ101 stops growing when the initial nitrate concentration increases up to 540 mg/L [28]. Furthermore, the maximum OD600 increases from 1.19 at 100 mg/L to 1.54 at 1000 mg/L, indicating that strain ZYL could grow well in this wide range of nitrate concentration. As a result, strain ZYL is able to tolerate high concentration of nitrate, suggesting its great application potential in the treating wastewater containing high concentration of nitrate.
Aerobic denitrification by strain ZYL
The denitrifying performance of strain ZYL was investigated under aerobic culture condition with nitrate as the sole N-source. As illustrated in Fig. 3a, there is no obvious change in microbial growth and nitrate concentration in the initial 6 h. Subsequently, the bacterial density increases rapidly and gradually stabilizes to reach the peak of OD600 of 1.19 with a fastest growth rate of 0.086 h−1. At the same time, strain ZYL decreases nitrate dramatically from 90.32 to 50.46 mg/L within 6 h, indicating that the nitrate reduction is closely related to the bacterium growth. The nitrate degradation efficiency achieves 100% at 36 h, and the average nitrate degradation rate is 2.80 mg/L/h, which is higher than those of Bacillus cereus GS-5 (2.69 mg/L/h) [16] and Pseudomonas putida ZN1 (2.38 mg/L/h) [15]. Moreover, the concentration of nitrite reaches a maximum of 1.40 mg/L at 18 h during the nitrate removal process, and then immediately drops to almost zero within 48 h. Besides, almost no accumulation of ammonium is detected during the whole denitrification process. In addition, the removal trend of COD and TN is similar to that of nitrate (Fig. 3a), and the maximum removal efficiencies of COD and TN are 96.15% and 98.45%, respectively, indicating that nitrogen and organic carbon are simultaneously degraded.
To further study the aerobic denitrification ability of strain ZYL, the denitrifying intermediate nitrite (102.97 mg/L) was utilized as the sole N-source. As shown in Fig. 3b, no significant increase in bacterial density is observed in the initial 6 h, demonstrating strain ZYL enters the lag phase. Subsequently, the cell growth of strain ZYL increases rapidly from 0.03 at 6 h to 1.18 at 48 h. Meanwhile, the content of nitrite decreases rapidly from 92.10 mg/L at 6 h to 0.02 mg/L at 36 h with the corresponding nitrite degradation efficiency of 99.98%. The average nitrite degradation rate reaches 2.86 mg/L/h, which is much higher than Pseudomonas putida ZN1 (1.97 mg/L/h) [15]. All these show that the removal of nitrite is closely related to strain ZYL’s growth. In addition, the removal characteristics of COD and TN are in accordance with the removal trend of nitrite, and the corresponding maximum removal efficiencies are 96.00% and 96.18%, respectively. Moreover, there is no significant accumulation of nitrate nitrogen and ammonium nitrogen during the whole nitrite removal process. All the above results demonstrate that strain ZYL can not only utilize nitrate, but also make full use of nitrite as the sole N-source for aerobic denitrification, which is similar to Marinobacter strain NNA5 [29] and Pseudomonas stutzeri YZN-001 [30].
Heterotrophic nitrification by strain ZYL
Generally speaking, the aerobic denitrifying bacteria usually have the ability for heterotrophic nitrification, so the heterotrophic nitrification performance of strain ZYL was investigated with ammonium as the sole N-source under aerobic culture condition. As shown in Fig. 3c, the bacterial density increases rapidly from 0.01 at 0 h to 1.12 at 24 h, and then stabilizes to reach the peak of OD600 of 1.17 at 48 h with a fastest growth rate of 0.093 h−1. Meanwhile, ammonium indicates a fast decrease from 98.35 to 9.25 mg/L in 24 h with a maximum ammonium removal rate of 4.74 mg/L/h between 12 and 18 h, and this removal rate is significantly higher than Diaphorobacter polyhydroxybutyrativorans (3.32 mg/L/h) [31], Klebsiella pneumoniae CF-S9 (4.30 mg/L/h) [32] and Rhodococcus sp. CPZ24 (3.10 mg/L/h) [33]. After 48 h incubation, 98.49% of ammonium is finally removed by the strain and the average removal rate of ammonium is 2.02 mg/L/h. During the whole heterotrophic nitrification process, the degradation efficiency of COD is 88.87%, which is much lower than that in the denitrifying process with nitrate (96.15%) and nitrite (96.00%) as the sole N-source. All these suggested strain ZYL consumes less carbon in its heterotrophic nitrification process than its aerobic denitrifying process. As a result, strain ZYL performs good ability for heterotrophic nitrification with ammonium as the sole N-source.
Nitrogen removal by strain ZYL with ammonium and nitrate as mixed N-sources
To further study the simultaneous nitrification and denitrification capability of strain ZYL, ammonium nitrogen (48.75 mg/L) and nitrate nitrogen (51.05 mg/L) were utilized as mixed N-sources, and the results are shown in Fig. 4. The OD600 increases rapidly and reaches the peak value of 1.25 at 48 h with the maximum growth rate of 0.093 h−1, which is higher than that with nitrate as the sole N-source. Compared with the denitrification with nitrate as the sole nitrogen source, there is almost no lag phase when both ammonium and nitrate were used as the mixed nitrogen sources, suggesting that strain ZYL grows well in the mixed N-sources.
For the removal of nitrogen in the mixed nitrogen source culture, ammonium is drastically reduced within 24 h, and its maximum removal efficiency and rate are 97.46% and 2.16 mg/L/h, respectively. However, the content of nitrate does not change in the initial incubation time of 12 h, and then rapidly decreases from 50.62 mg/L at 12 h to 0 mg/L at 36 h with a maximum nitrate degradation rate of 5.80 mg/L/h. All the above results show that strain ZYL can use ammonium preferentially in the mixed nitrogen sources, probably is due to the enzyme of ammonium oxidation in strain ZYL easier expresses than that of nitrate reduction, but the maximum removal rate of nitrate under this condition is much higher than that of ammonium, probably because the substrate competition between ammonium and nitrate results in the slow degradation of ammonium. The accumulation of other nitrogen compounds is hardly observed except for the original nitrogen source in the mixed nitrogen source removal process. Moreover, the degradation trend of TN is basically consistent with the sum of ammonium and nitrate, and the maximum degradation efficiency of TN is 98.88%. During the whole process, the concentration of COD gradually decreases with the growth of strain ZYL, and the maximum removal efficiency reaches 92.10%, which is lower than nitrate as the sole N-source, probably because the nitrification process consumes less energy than the denitrifying process.
Aerobic nitrate removal pathway by strain ZYL
The present study demonstrates that nitrate can be utilized as the sole N-source for growth by strain ZYL without accumulation of intermediates such as nitrite and ammonium. Moreover, N2 is observed in gas chromatography during the nitrate removal process. All these further indicate strain ZYL’s aerobic denitrification ability. To further verify its denitrification ability, the nitrogen balance experiment was conducted with nitrate as the sole N-source. In the process of nitrate reduction under aerobic conditions, 62.23% of the initial nitrate (99.72 mg/L) is converted to intracellular nitrogen to maintain bacterial growth, and 29.33% is converted into N2 in the system (Table 1). Approximately 4.61% of the initial nitrate is lost during this nitrate removal process, which might be due to the other removal form of gaseous nitrogen or may be attributed to the measurement errors caused by different analytical methods. As a result, strain ZYL can convert nitrate to N2, further proving its aerobic denitrifying ability.
To investigate the aerobic nitrate removal pathway of the isolate, the activities of NR and NIR were determined under aerobic culture conditions. Figure 5 indicates that there are NR and NIR in the crude cell during the nitrate removal process. It is well known that NR and NIR are two key enzymes involved in the denitrification process. Moreover, it has reported that periplasmic NR and cd1-type NIR are responsible for the aerobic conversion of nitrate to nitrite and nitrite to NO, respectively [34]. For strain ZYL, both NR and NIR show similar changing trend, and their activities increase first and then decrease with the nitrate removal. Moreover, the specific enzyme activity of NR is comparable to that of NIR in the nitrate removal process, and the maximum specific enzyme activities of NR and NIR are 0.16 and 0.15 U/mg proteins, respectively. For the isolate in our present study, the activity of NR is much higher than Acinetobacter sp. JR1 (0.034 U/mg proteins) [9], but lower than Acinetobacter sp. Y16 (0.43 U/mg proteins) [35]. The activity of NIR is higher than Paracoccus versutus LYM (0.0559 U/mg proteins) [36] and Bacillus cereus GS-5 (0.043 U/mg proteins) [16]. Thus, the detection of NR and NIR further demonstrates the aerobic denitrifying capacity of strain ZYL.
Figure 6a shows that both napA and nirS are successfully amplified in strain ZYL with the length of 763 bp and 1033 bp, respectively, and the BLAST results demonstrate that the napA and nirS from strain ZYL show a relatively close phylogenetic relationship to the napA and nirS from other Acinetobacter strains (Fig. 6b, c). The sequences of the two genes are submitted to GenBank with accession numbers MK944077 and MK944076 for napA and nirS, respectively. All these results further demonstrate the aerobic denitrifying capacity of strain ZYL. As we all know, the napA gene encodes the large catalytic subunit of NAP, belonging to the nap gene cluster [3]. The NAP in strain ZYL locating in the periplasmic compartment is not dependent on nitrate transport through the cytoplasmic membrane and thus catalyzes the first step in aerobic denitrification [37]. It has also been reported that napA is not sensitive to oxygen and can be expressed under aerobic conditions [38], further accounting for the good aerobic denitrifying capacity of strain ZYL under high dissolved oxygen. Moreover, it has been suggested that NAP shows a high affinity for nitrate [39], probably leading to the high nitrate removal of strain ZYL. The oxygen tolerance of nirS is of great importance to aerobic denitrification, and many researches indicate that nirS is more likely to be present in aerobic denitrification, such as Acinetobacter sp. JR1 [9], Pseudomonas putida ZN1 [15] and Bacillus cereus GS-5 [16]. As a result, the successful amplification of denitrifying genes napA and nirS further provides new proof for the aerobic nitrate removal pathway of strain ZYL.
a The amplification of napA and nirS genes of Acinetobacter haemolyticus ZYL. Marker: DL 5000 DNA Marker (TaKaRa, Japan). Phylogenetic trees of Acinetobacter haemolyticus ZYL based on napA (b) and nirS (c) gene sequences show the phylogenetic relationship of ZYL with other strains. Bootstrap values of 1000 replications are indicated at the interior branches
In general, both nitrate and nitrite can be used by strain ZYL under aerobic condition. In addition, N2 is observed in the gas with nitrate as the sole nitrogen source. According to nitrogen utilization, nitrogen balance analysis, enzyme assay and denitrifying gene amplification, there might be two aerobic metabolic pathways for nitrate removal in strain ZYL. One is that nitrate is mainly utilized for cell synthesis through assimilation, the other is that nitrate is reduced to N2 via nitrite, which is consistent with the pathway of Pseudomonas stutzeri ZF31 [19] and Pseudomonas stutzeri XL-2 [26].
Conclusions
Acinetobacter haemolyticus ZYL was a new isolated bacterium from domestic wastewater sludge. Its optimum conditions for nitrate removal under aerobic condition were sodium succinate as carbon source, C/N 20, pH 7.0, 30 °C and 120 rpm. It exhibited good abilities for aerobic denitrification and heterotrophic nitrification. Moreover, ammonium was preferred to be utilized by strain ZYL during the simultaneous nitrification and denitrification process. Further studies demonstrated that nitrate was assimilated directly by the isolate for cell synthesis and also converted into N2 through aerobic denitrification. All the results show that strain ZYL has great potential in the treatment of actual nitrogen-containing wastewater with high C/N, such as food processing wastewater.
References
Ahn Y-H (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721
Habermeyer M, Roth A, Guth S, Diel P, Engel K-H, Epe B, Fürst P, Heinz V, Humpf H-U, Joost H-G, Knorr D, de Kok T, Kulling S, Lampen A, Marko D, Rechkemmer G, Rietjens I, Stadler RH, Vieths S, Vogel R, Steinberg P, Eisenbrand G (2015) Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol Nutr Food Res 59:106–128
Ji B, Yang K, Zhu L, Jiang Y, Wang H, Zhou J, Zhang H (2015) Aerobic denitrification: a review of important advances of the last 30 years. Biotechnol Bioprocess Eng 20:643–651
Zhang Y, Shi Z, Chen M, Dong X, Zhou J (2015) Evaluation of simultaneous nitrification and denitrification under controlled conditions by an aerobic denitrifier culture. Bioresour Technol 175:602–605
Robertson LA, Kuenen JG (1984) Aerobic denitrification: a controversy revived. Arch Microbiol 139:351–354
He T, Li Z, Sun Q, Xu Y, Ye Q (2016) Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour Technol 200:493–499
Zheng Z, Zhang D, Li W, Qin W, Huang X, Lv L (2018) Substrates removal and growth kinetic characteristics of a heterotrophic nitrifying-aerobic denitrifying bacterium, Acinetobacter harbinensis HITLi7T at 2 °C. Bioresour Technol 259:286–293
Ma Q, Cai Y, He Z (2019) Complete genome sequence of a novel aerobic denitrifying strain, Pseudomonas monteilii CY06. Mar Genom 47:100661
Yang J-R, Wang Y, Chen H, Lyu Y-K (2019) Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour Technol 274:56–64
Zhao B, He YL, Hughes J, Zhang XF (2010) Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour Technol 101:5194–5200
Ren Y-X, Yang L, Liang X (2014) The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour Technol 171:1–9
Ahmad WA, Ahmad WH, Karim NA, Raj AS, Zakaria ZA (2013) Cr(VI) reduction in naturally rich growth medium and sugarcane bagasse by Acinetobacter haemolyticus. Int Biodeterior Biodegrad 85:571–576
Lee M, Woo S-G, Ten LN (2012) Characterization of novel diesel-degrading strains Acinetobacter haemolyticus MJ01 and Acinetobacter johnsonii MJ4 isolated from oil-contaminated soil. World J Microbiol Biotechnol 28:2057–2067
Bihari Z, Pettkó-Szandtner A, Csanádi G, Balázs M, Bartos P, Kesserű P, Kiss I, Mécs I (2007) Isolation and characterization of a novel n-alkane-degrading strain, Acinetobacter haemolyticus AR-46. Zeitschrift für Naturforschung C 62:285–295
Zhang N, Chen H, Lyu Y, Wang Y (2019) Nitrogen removal by a metal-resistant bacterium, Pseudomonas putida ZN1, capable of heterotrophic nitrification–aerobic denitrification. J Chem Technol Biotechnol 94:1165–1175
Rout PR, Bhunia P, Dash RR (2017) Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour Technol 244:484–495
Guo L, Chen Q, Fang F, Hu Z, Wu J, Miao A, Xiao L, Chen X, Yang L (2013) Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Bioresour Technol 142:45–51
Zheng H-Y, Liu Y, Gao X-Y, Ai G-M, Miao L-L, Liu Z-P (2012) Characterization of a marine origin aerobic nitrifying–denitrifying bacterium. J Biosci Bioeng 114:33–37
Huang T, Guo L, Zhang H, Su J, Wen G, Zhang K (2015) Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, Pseudomonas stutzeri strain ZF31, isolated from a drinking-water reservoir. Bioresour Technol 196:209–216
Luo X, Su J, Shao P, Liu H, Luo X (2018) Efficient autotrophic denitrification performance through integrating the bio-oxidation of Fe(II) and Mn(II). Chem Eng J 348:669–677
Zhang H, Zhao Z, Kang P, Wang Y, Feng J, Jia J, Zhang Z (2018) Biological nitrogen removal and metabolic characteristics of a novel aerobic denitrifying fungus Hanseniaspora uvarum strain KPL108. Bioresour Technol 267:569–577
Zhang W, Yan C, Shen J, Wei R, Gao Y, Miao A, Xiao L, Yang L (2019) Characterization of aerobic denitrifying bacterium Pseudomonas mendocina strain GL6 and its potential application in wastewater treatment plant effluent. Int J Environ Res Public Health 16:364
Huang F, Pan L, Lv N, Tang X (2017) Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification–aerobic denitrification. J Biosci Bioeng 124:564–571
López-Maury L, Marguerat S, Bähler J (2008) Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 9:583–593
Song ZF, An J, Fu GH, Yang XL (2011) Isolation and characterization of an aerobic denitrifying Bacillus sp. YX-6 from shrimp culture ponds. Aquaculture 319:188–193
Zhao B, Cheng DY, Tan P, An Q, Guo JS (2018) Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour Technol 250:564–573
Patureau D, Bernet N, Delgenès JP, Moletta R (2000) Effect of dissolved oxygen and carbon–nitrogen loads on denitrification by an aerobic consortium. Appl Microbiol Biotechnol 54:535–542
Idi A, Ibrahim Z, Mohamad SE, Majid ZA (2015) Biokinetics of nitrogen removal at high concentrations by Rhodobacter sphaeroides ADZ101. Int Biodeterior Biodegrad 105:245–251
Liu Y, Ai G-M, Miao L-L, Liu Z-P (2016) Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour Technol 206:9–15
Zhang J, Wu P, Hao B, Yu Z (2011) Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresour Technol 102:9866–9869
Zhang S, Sun X, Fan Y, Qiu T, Gao M, Wang X (2017) Heterotrophic nitrification and aerobic denitrification by Diaphorobacter polyhydroxybutyrativorans SL-205 using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) as the sole carbon source. Bioresour Technol 241:500–507
Padhi SK, Tripathy S, Sen R, Mahapatra AS, Mohanty S, Maiti NK (2013) Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int Biodeterior Biodegrad 78:67–73
Chen P, Li J, Li QX, Wang Y, Li S, Ren T, Wang L (2012) Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24. Bioresour Technol 116:266–270
Richardson DJ, Watmough NJ (1999) Inorganic nitrogen metabolism in bacteria. Curr Opin Chem Biol 3:207–219
Huang X, Li W, Zhang D, Qin W (2013) Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification–aerobic denitrification at low temperature. Bioresour Technol 146:44–50
Shi Z, Zhang Y, Zhou J, Chen M, Wang X (2013) Biological removal of nitrate and ammonium under aerobic atmosphere by Paracoccus versutus LYM. Bioresour Technol 148:144–148
Kraft B, Strous M, Tegetmeyer HE (2011) Microbial nitrate respiration—genes, enzymes and environmental distribution. J Biotechnol 155:104–117
Reyes F, Roldán MD, Klipp W, Castillo F, Moreno-Vivián C (1996) Isolation of periplasmic nitrate reductase genes from Rhodobacter sphaeroides DSM 158: structural and functional differences among prokaryotic nitrate reductases. Mol Microbiol 19:1307–1318
Sparacino-Watkins C, Stolz JF, Basu P (2014) Nitrate and periplasmic nitrate reductases. Chem Soc Rev 43:676–706
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant no. 51778397), the International Cooperation Projects of Shanxi Province (Grant no. 201603D421040), and the Key Research and Development Program of Shanxi Province (Grant no. 201903D311004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Zou, YL., Chen, H. et al. Nitrate removal performances of a new aerobic denitrifier, Acinetobacter haemolyticus ZYL, isolated from domestic wastewater. Bioprocess Biosyst Eng 44, 391–401 (2021). https://doi.org/10.1007/s00449-020-02451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02451-0