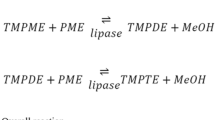Abstract
LML-type structured lipids are one type of medium- and long-chain triacylglycerols. LML was synthesized using immobilized Talaromyces thermophilus lipase (TTL)-catalyzed interesterification of tricaprylin and ethyl linoleate. The resin AB-8 was chosen, and the lipase/support ratio was determined to be 60 mg/g. Subsequently, the immobilized TTL with strict sn-1,3 regiospecificity was applied to synthesize LML. Under the optimized conditions (60 °C, reaction time 6 h, enzyme loading of 6% of the total weight of substrates, substrate of molar ratio of ethyl linoleate to tricaprylin of 6:1), Triacylglycerols with two long- and one medium-chain FAs (DL-TAG) content as high as 52.86 mol% was obtained. Scale-up reaction further verified the industrial potential of the established process. The final product contained 85.24 mol% DL-TAG of which 97 mol% was LML after purification. The final product obtained with the high LML content would have substantial potential to be used as functional oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medium-chain TAGs (MCTs) were found as low-calorie oil to remittence the fat accumulation of human being [1]. Compared with the conventional edible oils, consumption of medium-chain TAGs produces large amounts of medium-chain FAs which would be directly transported into the liver through the portal vein to supply energy, rather than being transported to the adipose tissue for storage. However, it has been demonstrated that the long-term ingestion of medium-chain TAGs can cause gastrointestinal problems, such as gastric discomfort, cramps, nausea, abdominal pain, and diarrhea [2, 3]. Most importantly, medium-chain TAGs cannot provide essential FAs for humans. Alternatively, medium- and long-chain TAGs (MLCTs) combine the advantages of both the medium-chain TAGs and long-chain TAGs, which can not only suppress the accumulation of body fat but can also serve as essential FAs [4,5,6]. Therefore, there is a growing demand for the MLCTs in recent years due to the increasing health concern of diabetes, ischemic heart diseases, and some cancers that are primarily caused by obesity [7, 8].
MLCTs contain six types of TAG, including MLL, LML, LLM, MML, LMM, and MLM, and MLM has been extensively studied for its use as a low-calorie TAG [9,10,11]. LML is one type of MLCTs that has long-chain FAs at the sn-1,3 positions and medium-chain FAs at the sn-2 position. LML is superior to the other two types of medium- and long-chain TAGs (i.e., MML and LMM) for cooking and frying applications, because it has higher smoke point and boiling point than MML and LMM. The LML would be cleaved to long-chain FAs and 2-monoacylglycerol (2-MAG) with medium-chain FAs after ingestion. Compared with the 2-MAG containing long-chain FAs produced by the ingestion of conventional edible oils, the 2-MAG with medium-chain FAs is poor substrates for the re-synthesis of TAG in the intestinal epithelial cell; consequently, the re-synthesis of TAG would be greatly inhibited, and most of the 2-MAG and FAs produced would be transported to the liver through the portal vein to supply energy or for other purposes [12, 13]. The unique metabolic pathway distinguished from the conventional edible oils enables the LML to exhibit beneficial effects on humans. However, few reports have been published to date regarding the synthesis of LML. The interesterification of soybean oil and MCTs using Novozym 435 has been used to synthesize MLCTs [14], but LML level in the MLCTs products containing MLL, LML, LLM, and MML was low. In addition, a two-step chemoenzymatic route, which consists the incorporation of long-chain FAs into the sn-1,3 positions of TAGs using immobilized CLA-B lipase and the introduction of medium-chain FAs into the sn-2 position of TAGs using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, has been developed to synthesize LML-type TAGs [15]. In this context, an environmentally friendly and simple approach is highly needed to produce LML with high purity.
The enzymatic synthesis of structured lipids has gained widespread attention due to their mild reaction conditions, high specificity, and environmentally friendliness [16, 17]. Typically, lipases with sn-1,3 regiospecificity, such as Lipozyme RM IM and immobilized Rhizopus oryzae lipase, are used to synthesize ABA (TAGs with the same FAs at the sn-1 and sn-3 positions, but with different FAs at the sn-2 position) type structured lipids by acidolysis or interesterification using FAs or FA ethyl esters as an acyl donor [18,19,20,21]. In particular, Lipozyme RM IM is the only commercial immobilized lipase with the strict sn-1,3 regiospecificity to produce ABA-type structured lipids. To achieve ABA-type structured lipids with high purity, further exploration of novel lipase resources with strict sn-1,3 regiospecificity to synthesize ABA-type structured lipids is of great importance in the modification of fats and oils.
Talaromyces thermophilus lipase (TTL) is a thermostable lipase and has been proven to have great potential to use in laundry detergents and biodiesel production [22,23,24]. Although TTL showed excellent activity in hydrolytic and methanolytic reactions, its type of performance in other types of reactions is still unknown. Our preliminary studies found that TTL was a lipase with strict sn-1,3 regiospecificity, which may bepotential to produce LML products with high purity.
Therefore, in this study, TTL was immobilized and utilized to synthesize LML by interesterification of tricaprylin and ethyl linoleate to establish an industrial potential process. First, five resins were examined for their capability to immobilize TTL followed by the optimization of the immobilization process using the selected carrier. Subsequently, the immobilized TTL was characterized and was used to synthesize LML. Significant variables, such as the reaction temperature, enzyme loading, substrate molar ratio, and reaction time, were chosen to study their effects on the interesterification. In addition, a scale-up interesterification reaction was performed under the optimized conditions to evaluate the industrial potential of the established process. Finally, the produced LML was purified using molecular distillation and characterized by the analysis of its FA composition.
Materials and methods
Materials
The resins AB-8 and DA201 were kindly provided by the Chemical Plant of Nankai University (Tianjin, China) and Zhengzhou Qinshi Technology Co., Ltd. (Henan, China), respectively. The resins ECR1030, SA-1, and HP2MGL were purchased from Rohm and Haas (Philadelphia, USA). Bovine serum albumin was purchased from Shanghai Bio Science and Technology Company (Shanghai, China). The Bradford reagent was purchased from Sigma-Aldrich (Wuhan, China). n-Hexane, 2-propanol, and formic acid were purchased from the Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Triolein (> 99%), the standards of TAG, DAG (15% of 1,2-DAG and 85% of 1,3-DAG), MAG, tricaprylin (> 99%), and ethyl linoleate were purchased from Sigma-Aldrich (Shanghai, China). Commercial medium- and long-chain TAGs were purchased from the Baili Pharmaceutical Industry Co., Ltd. (Chengdu, China). All the other reagents and chemicals were of high purity and analytical grade.
High cell density fermentation of TTL
The transformed strain showing the highest lipase activity in shake-flask culture was cultivated in a high cell density fermenter. High cell density fermentation was conducted in a 50 L bioreactor (Baoxing Co., Shanghai, China). The inoculum was cultured in BMGY media. The cells were grown for 18–20 h at 30 °C on a shaker at 200 rpm. 10% (v/v) of the inoculum was inoculated into a 50 L bioreactor containing 20 L basal salt media comprised of 0.47 g/L CaSO4·2H2O, 9.1 g/L K2SO4, 7.5 g/L MgSO4·7H2O, 6.2 g/L KOH, 13.35 mL/L H3PO4 (85%), 20.0 g/L glycerol and 1.5 mL Pichia trace metal 1 (PTM1) (Guangzhou Chemical Reagent Factory, Guangzhou, China). 1-L PTM1 consists of 6 g CuSO4·5H2O, 0.08 g NaI, 3 g MnSO4·H2O, 0.5 g CoCl2, 20 g ZnCl2, 0.02 g H3BO3, 0.2 g Na2MoO4·2H2O, 65 g FeSO4·7H2O, 0.2 g biotin, and 30 mL 6 N H2SO4. The temperature was controlled at 30 °C, and the pH was maintained at 5.0 using NH4OH (28%) and H3PO4 (10%). The agitation rate was set at 500 rpm, and the aeration rate was 40 L/min. When the glycerol had been consumed, as indicated by an increase in the dissolved oxygen, 0.5% (v/v) methanol was added to induce the expression of the lipase. The feeding of the methanol was linked to the DO. When the initial methanol was depleted (indicated by an abrupt increase in the DO), 80 g of 100% methanol solution containing 1.2% (v/v) PTM1 was automatically added. The concentration of methanol was kept stable by monitoring the dissolved oxygen content and maintaining it higher than 20%.
Immobilization of the TTL
The resultant fermentation broth of the TTL was centrifuged, and then, the supernatant containing TIL was used directly to immobilize TIL using the treated macroporous resins. The selected macroporous resins (Table 1) were treated as described by Wang et al. to remove the bubbles, residual monomers, and compounds from the hole of the resins [25]. Briefly, 9 g of treated resin was added to a 1-L conical flask and mixed with 105 mL of the supernatant containing TTL (60 mg protein per g resin) and 105 mL of 20 mM pH 7.0 phosphate buffer. Subsequently, the mixture was shaken at 30 °C for 8 h using an air bath orbital shaker (180 rpm). After immobilization, the immobilized TTL on the resin was recovered using a Buchner funnel and washed with 20 mM pH 7.0 phosphate buffer until no protein could be detected in the eluent. The immobilized TTL recovered was dried in a vacuum desiccator for 8 h to remove water from the immobilized TTL. The immobilization performance was evaluated using the protein loading [26], hydrolytic activity [27], and specific activity. The TTL-immobilized carrier with the highest hydrolytic and specific activities was selected to optimize the immobilization experiment. Briefly, the effect of the lipase/resin ratio (20–80 mg/g) on the immobilization of TTL was studied. The best TTL-immobilized resin was used to characterize and perform enzymatic reactions.
Regiospecificity of the immobilized TTL
The regiospecificity of the immobilized TTL was analyzed using the hydrolysis of triolein as described by Li et al. [27] with minor modifications. Briefly, 1-g triolein was added to a 10-mL conical flask and mixed with 0.2-mL 20-mM pH 7.0 phosphate buffer. The flask was incubated in a glycerol bath at 40 °C. The reaction was initiated by the addition of 50 U immobilized TTL. Each sample (30 µL) was withdrawn at periodic intervals and mixed with 1 mL mobile phase (n-hexane, 2-propanol, and formic acid = 21:1:0.003, by volume). Subsequently, 0.5 g anhydrous sodium sulfate was added to the mixture and mixed by vortexing, followed by centrifugation at 10,000×g for 2 min. The resultant supernatant (0.8 mL) was withdrawn and transferred into a 2-mL chromatographic tube for acylglycerol and FA profiles analysis using normal phase HPLC (NP-HPLC, refractive index detector, Waters Corporation, Milford, MA, USA) using a Phenomenex Luna column (4.6 mm i.d. × 250 mm, 5 µm particle size, Phenomenex Corporation, Torrance, CA, USA). The mobile phase was a mixture of n-hexane, 2-propanol and methanoic acid (21:1:0.003, v/v/v), and the solution was eluted at 30 °C with a flow rate of 1 mL/min. The positional specificity index was calculated to evaluate the regiospecificity of the immobilized TTL as described by Ota et al. [28].
Synthesis of LML by immobilized TTL-catalyzed interesterification
A 50-mL conical flask containing tricaprylin and ethyl linoleate was incubated in a silicon oil bath at the desired temperature. The reaction was initiated with the addition of the immobilized TTL. During interesterification, the reaction temperature (50, 55, 60, 65, and 70 °C), enzyme loading (2%, 4%, 6%, 8%, and 10%, by weight of the total substrates), and the molar ratio of ethyl linoleate to tricaprylin (1:1, 2:1, 3:1, 4:1, 5:1, 6:1, and 7:1) were varied to investigate their effects on the interesterification. Samples (20 µL) were withdrawn at selected time points (0, 1, 3, 6, 9, 12, and 24 h) and mixed with 1 mL n-hexane. Subsequently, the mixture was filtered through a 0.22-µm nylon membrane, and approximately, 0.8 mL filtered liquid was collected and analyzed on an Agilent 7890A GC equipped with a DB-1HT column (30 m × 0.25 mm × 0.2 µm) using nitrogen as the carrier gas with a flow rate of 1.1 mL/min. The column temperature was initially held at 200 °C for 2 min before being programmed to reach 300 °C at a rate of 10 °C/min and was maintained isothermally for 15 min. The temperatures for the injector and detector were set at 250 and 280 °C, respectively. A split ratio of 20:1 was used.
Scale-up reaction
To evaluate the potential industrial application of the process established to synthesize of LML, a scale-up reaction of about 300-fold was conducted using the optimized conditions (reaction temperature of 60 °C, enzyme loading of 6% and tricaprylin/ethyl linoleate molar ratio of 1:6). The total weight of the tricaprylin and ethyl linoleate was 3 kg. The reaction was performed in a 5-L three-neck round bottom flask and stirred using a mechanical agitator (IKA RW20) with an agitation speed of 200 rpm. The reaction was initiated after the addition of the immobilized TTL. Samples (20 µL) were withdrawn at selected timepoints (1, 3, and 6 h) and prepared for GC analysis as described above. The reaction was stopped after 6 h, and the reaction mixture was collected for the subsequent purification.
Purification of the final product using short-path molecular distillation
A two-stage molecular distillation (MD-S80 short-path falling film distiller, Guangzhou Hanwei Co., Ltd., Guangzhou, China) was applied to purify the DL-TAG from the scale-up reaction to remove the undesirable products produced during interesterification. The conditions for the first stage of molecular distillation were as follows: a feeding temperature of 60 °C, a feeding flow rate of 2 g/min, an evaporating temperature of 100 °C, a pressure of 10 Pa, and a scraper speed of 300 rpm. The conditions for the second stage of molecular distillation were as follows: a feeding flow rate of 1.5 g/min, an evaporation temperature of 200 °C, a scraper speed of 300 rpm, a pressure of 1 Pa, and a condenser temperature of 40 °C. The fraction enriched with DL-TAG was collected for TAG composition and FA composition analyses.
Analysis of the interesterification products using GC
The interesterification products were analyzed using GC equipped with a flame ionization detector and filtered with a DB-1HT column (30 m × 0.25 mm, i.d., 0.1 µm). A temperature program was established to separate ethyl caprylate, ethyl palmitate, tricaprylin, SL-TAG(Triacylglycerols with one long- and two medium-chain FAs), DL-TAG, and trilinolein. First, the oven temperature was held at 200 °C for 2 min, programmed to 300 °C at a rate of 10 °C/min, and maintained for 15 min. Subsequently, the oven temperature continued to elevate at a rate of 5 °C/min to 330 °C and was held for 15 min. The carrier gas was nitrogen and the split ratio was 20:1. Each peak in the chromatogram was identified by comparison with the corresponding standard. In this study, the relative molar content of DL-TAG was expressed as the molar ratio of DL-TAG molar content to the sum molar content of tricaprin, SL-TAG, DL-TAG, and trilinolein.
Analysis of the FA composition and distribution of the final product
Acyl migration may occur during immobilized TTL-catalyzed interesterification. Therefore, the DL-TAG produced may contain not only LML but also LLM and MLL. To determine the purity of the LML in the final product, the FA composition at the sn-2 position of the final product was analyzed. The final product was hydrolyzed using pancreatic lipase as described by Sahin et al. [29]. The 2-MAG produced was identified and scraped off a thin layer chromatography plate. Subsequently, the scraped 2-MAG was methylated as described by Wang et al. [30]. The methylated 2-MAG was analyzed using GC as previously described by our group [31]. In addition, approximately 20 mg of the final product was methylated followed by analysis of the total FA composition using GC.
Statistical analysis
All the reactions were performed in triplicate, and the data are shown as the means with the standard deviations. Differences between the measured values were assessed by a two-tailed Student’s test at p < 0.05.
Results and discussion
Immobilization of TTL
Screening of the carrier to immobilize TTL
In this study, five carriers with different particle sizes, specific surface areas, and pore diameters were screened for their abilities to immobilize TTL. As shown in Table 1, the hydrolytic activity of 5588.25 U/g and the specific activity of 58.2 U/mg of the immobilized TTL using AB-8 as a carrier were the highest among all the immobilized preparations obtained. Although resin SA-1 had the highest protein load (99.17 mg/g), it did not give the highest hydrolytic activity (2982 U/g) and specific activity (30.07 U/mg) of the immobilized TTL. The results indicate that the activity of immobilized lipase is associated with the characteristics of carries including the particle size, specific surface area, and pore diameter of the carrier [32, 33]. AB-8 is a kind of nonpolar resin, while other resins are polar or weakly polar. The hydrophobic interactions between AB-8 resins and TTL may favor the opening of the lid of TTL, thus promoting the exposure of the catalytic site of TTL. As a consequence, a relatively high activity of the immobilized lipase using AB-8 was obtained. Further studies are still needed to clarify the detailed interaction mechanisms. Therefore, the AB-8 resin was chosen to immobilize the TTL due to the highest specific activity of the immobilized TTL.
Effect of the lipase/support ratio on the immobilization of TTL
To identify a more suitable and economical condition to immobilize TTL, the effect of lipase/support ratios ranging from 20 to 80 mg/g on the immobilization of TTL using AB-8 as the carrier was studied, and the results are shown in Fig. 1. As expected, the protein loading increased from 39.58 to 113.23 mg/g with an increasing lipase/support ratio from 20 to 80 mg/g. The hydrolytic activity of the immobilized TTL reached the maximum of 5588.25 U/g at the lipase/support ratio of 60 mg/g and began to decrease. However, the specific activity of the immobilized TTL decreased as the lipase/support ratio increased, which indicated that the contact between a substantial amount of adsorbed lipases on the carrier and the substrates was restricted. Our previous study found that CLA-B distributed not only on the surface of the carrier, but also inside of the carrier during immobilization [34]. The lipase distribution of a carrier may affect the contact between the substrate and the lipase. Considering that the highest hydrolytic and moderate specific activities could be obtained at a lipase/support ratio of 60 mg/g, the lipase/support ratio of 60 mg/g was selected to immobilize TTL. Under the optimized conditions (lipase/support ratio = 60 mg/g), immobilized TTL was produced on large scale. The immobilized TTL with a hydrolytic activity of 5614.36 U/g and a specific activity of 58.33 U/mg was preserved in refrigerator at 4 °C prior to use.
Regiospecificity of the immobilized TTL
The regiospecificity of immobilized TTL was studied by monitoring the hydrolysis of triolein, and the results are shown in Fig. 2. The 1(3),2-diacylglycerols (DAG) and free FA increased as the hydrolysis reaction proceeded. The MAG increased at a relatively slower rate. Interestingly, the 1,3-DAG was undetectable at the initial 9 min and was 0.06% after 10 min. The low content of 1,3-DAG and the high content of 1(3),2-DAG in the reaction mixture indicated that the immobilized TTL preferred to act at the sn-1 and sn-3 positions of the glycerol backbone rather than at the sn-2 position. After 10 min, the reaction mixture contained 59.74% TAG, 20.61% 1(3),2-DAG, 0.06% 1,3-DAG, 16.74% FA, and 2.85% 2-MAG. The positional specificity index of the immobilized TTL was 98.84, which is significantly higher than the positional specificity index (93.3) of Lipozyme RM IM that is commonly known as an sn-1,3 specific lipase [28]. Overall, the results obtained demonstrated that immobilized TTL exhibited strict sn-1,3 regiospecificity towards TAG, which indicate a substantial potential to synthesize ABA-type structured lipids. Therefore, the potential of immobilized TTL to synthesize of LML was explored in the following studies.
Synthesis of LML by immobilized TTL-catalyzed interesterification
Effect of reaction temperature on the interesterification
The immobilized TTL with high activity and strict sn-1,3 regiospecificity was employed to synthesize the LML. Figure 3 shows the effects of temperature on the DL-TAG content. When the reaction temperature increased from 50 to 60 °C, the DL-TAG content increased from 31.46 mol% (24 h) to 40.34 mol% (24 h). The DL-TAG content began to decrease when the reaction temperature was higher than 60 °C. The increased DL-TAG content with increasing temperature to 60 °C may due to the favorable lipase activity at these temperatures. The DL-TAG content obtained at 65 °C (31.52 mol%) and 70 °C (31.57 mol%) was significantly lower than that obtained at 60 °C, which could be due primarily to the denaturation of the immobilized TTL at relatively higher temperatures. Therefore, the reaction temperature was fixed at 60 °C for the following experiments.
Effect of enzyme loading on the interesterification
The enzyme amount used determines the reaction rate and production costs. The effect of enzyme loading on the interesterification was evaluated, and the results are shown in Fig. 4. High enzyme loading favors a shorter reaction time to reach equilibrium. When the enzyme loading was 6%, the DL-TAG content reached 36.76 mol% (6 h), which was significantly higher than that obtained at an enzyme loading of 2% (12.16 mol%) or 4% (21.56%) at 6 h. When the enzyme loading was higher than 6%, the DL-TAG content was higher than 40.20 mol% at 6 h, which was almost equal with that obtained at enzyme loading of 2% and 4% after 24 h of reaction. Although the DL-TAG content obtained at an enzyme loading of 8% or 10% at the initial 6 h of reaction was significantly higher than that obtained at the enzyme loading of 6% at the same reaction time, there is no significant differences between the DL-TAG content after 9 h of reaction. The use of too much enzyme not only adds the production costs but can also influence the mass transfer of the reaction system. Thus, enzyme loading of 6% was selected for the following experiments.
Effect of substrate molar ratio on the interesterification
The effect of the ethyl linoleate/tricaprylin molar ratio on the interesterification was studied. As shown in Fig. 5, a high molar ratio of ethyl linoleate to tricaprylin favors a higher initial reaction rate. When the molar ratio was increased from 2:1 to 4:1, the DL-TAG content increased from 26.14 mol% (24 h) to 46.72 mol% (24 h). However, when the molar ratio was higher than 4:1, the DL-TAG content increased first and then decreased. When the molar ratio was 5:1, the highest DL-TAG content (49.31 mol%) was obtained at 12 h. When the molar ratio was 6:1, the highest DL-TAG content of 52.86 mol% was obtained at 6 h, and the DL-TAG content began to decrease. Although the highest DL-content could be obtained at a relatively shorter reaction time of 3 h at a molar ratio of 7:1, the highest DL-TAG content obtained at 7:1 (52.44 mol%) was slightly lower than that obtained at 6:1 (52.86 mol%). Therefore, the ethyl linoleate/tricaprylin molar ratio was chosen to be at 6:1, and the reaction time was determined at 6 h for the synthesis of DL-TAG.
As discussed above, the DL-TAG content began to decrease when the DL-content reached its maximum at molar ratios of 5:1, 6:1 and 7:1. The possible reasons for the decrease of the DL-content include the partial DL-TAG conversion to trilinolein.
The evaluation of the scale-up reaction and the purification of the final product
To assess the potential of the established process to synthesize of DL-TAG, a scale-up reaction of approximately 300-fold was performed under the optimized conditions. The reaction process was monitored by the determination of the contents of tricaprylin, SL-TAG, DL-TAG, and trilinolein, respectively. As illustrated in Fig. 6, the tricaprylin content decreased dramatically at the initial 1 h and then decreased slowly during the next 1 h. The tricaprylin content decreased very slowly after 2 h, which could be attributed to the low concentration of tricaprylin in the reaction mixture after 2 h and the equilibrium between the products. During the interesterification, the SL-TAG was first produced and the DL-TAG and trilinolein were subsequently produced. As expected, the SL-TAG content increased dramatically at the initial 1 h and began to decrease. However, the DL-TAG and trilinolein contents increased as the reaction proceeded. The final reaction mixture contained 5.67 mol% tricaprylin, 33.01 mol% SL-TAG, 53.19 mol% DL-TAG, and 8.13 mol% trilinolein.
The reaction mixture of the scale-up reaction was purified further using two-stage molecular distillation to remove the tricaprylin and SL-TAG. As shown in Table 2, the final product consisted of 0.97 mol% tricaprylin, 1.34 mol% SL-TAG, 85.24 mol% DL-TAG, and 12.44 mol% trilinolein after purification. The DL-TAG content rose to 85.24 mol% after purification.
FA composition analysis of the final product
The FA composition of the sn-2 position of the final product was analyzed to determine the purity of the LML in DL-TAG. As discussed above, the final product contained 85.24 mol% DL-TAG, 0.97 mol% tricaprylin, 1.34 mol% SL-TAG, and 12.44 mol% trilinolein. If acyl migration did not occur during interesterification, all the DL-TAG would be LML, and the highest LML content in the final product should be 85.24 mol%. As shown in Table 3, 85.01 mol% C8:0 occupied the sn-2 position of the final product. The 85.01 mol% C8:0 obtained at the sn-2 position of the final product was derived from tricaprylin (MMM), SL-TAG, and DL-TAG. If no acyl migration occurred, the 1.34 mol% SL-TAG obtained would only contain LMM or MML. Therefore, it can be concluded that at least 82.7 mol% (85.01 mol% minus 0.97 mol% and 1.34 mol%) C8:0 at the sn-2 position of the final product was derived from DL-TAG. In summary, the final product contained at least 82.7 mol% LML.
The total FA composition of the final product was also analyzed. The final product contained 30.28 mol% C8:0 and 68.24 mol% C18:2n6. The modified lipids with medium- and long-chain FAs combine the advantages of both medium-chain TAG and long-chain TAG. In summary, the final product obtained with at least 82.7 mol% LML may have substantial potential to be used as functional oils.
Conclusions
LML is one of the types of medium- and long-chain TAGs. However, to date, little information is available on the synthesis of LML. In summary, immobilized TTL with strict sn-1,3 regiospecificity was employed to synthesize LML using interesterification of tricaprylin and ethyl linoleate for the first time. A complete process including the immobilization of TTL, regiospecificity study of the immobilized TTL, optimization of the reaction process, scale-up reaction, and the purification of the product were studied in detail. Immobilized TTL exhibited superior activity during interesterification and 53.19 mol% DL-TAG was obtained under the optimized conditions. After purification, the final product with at least 82.7 mol% LML was obtained. The scale-up reaction showed that the established reaction process had substantial potential for industrial applications. In conclusion, this study broadens the application ranges of the TTL, and the immobilized TTL with strict sn-1,3 regiospecificity may have more potential applications in the oils and fats industry. Additionally, this study may provide insights for further research involving the production of LML-type structured lipids and the application of TTL.
Abbreviations
- TTL:
-
Talaromyces thermophilus lipase
- TAG:
-
Triacylglycerol
- FA:
-
Fatty acid
- MAG:
-
Monoacylglycerol
- DAG:
-
Diacylglycerol
References
St-Onge MP, Jones PJH (2002) Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr 132:329–332
Zhao HZ, Lu ZX, Bie XM, Lu FX, Liu ZM (2007) Lipase catalyzed acidolysis of lard with capric acid in organic solvent. J Food Eng 78:41–46
Marten B, Pfeuffer M, Schrezenmeir J (2006) Medium-chain triglycerides. Int Dairy J 16:1374–1382
Lu JY, Jin QZ, Wang XG, Wang XS (2017) Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system. Process Biochem 54:89–95
Matulka RA, Noguchi O, Nosaka N (2006) Safety evaluation of a medium- and long-chain triacylglycerol oil produced from medium-chain triacylglycerols and edible vegetable oil. Food Chem Toxicol 44:1530–1538
Koh SP, Tan CP, Lai OM, Arifin N, Yusoff MSA, Long K (2010) Enzymatic synthesis of medium- and long-chain triacylglycerols (MLCT): optimization of process parameters using response surface methodology. Food Bioprocess Techol 3:288–299
Mumme K, Stonehouse W (2015) Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Am Acad Dermatol 115:249–263
Zhou S, Wang Y, Jiang Y, Yu LL (2017) Safety assessment of medium- and long-chain triacylglycerols containing 30%(w/w) medium-chain fatty acids in mice and rats. Regul Toxicol Phar 86:42–48
Costa CM, Osório NM, Canet A, Rivera I, Sandoval G, Valero F, Ferreira-Dias S (2018) Production of MLM type structured lipids from grapeseed oil catalyzed by non-commercial lipases. Eur J Lipid Sci Technol 120:1700320
Mu H, Porsgaard T (2005) The metabolism of structured triacylglycerols. Prog Lipid Res 44:430–448
Morales-Medina R, Munio M, Guadix A, Guadix EM (2017) Development of an up-grading process to produce MLM structured lipids from sardine discards. Food Chem 228:634–642
Babayan VK (1987) Medium chain triglycerides and structured lipids. Lipids 22:417–420
Bach AC, Ingenbleek Y, Frey A (1996) The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res 37:708–726
Lu J, Jin QZ, Wang XG, Wang XS (2017) Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system. Process Biochem 54:89–95
Gudmundsdottir AV, Hansen KA, Magnusson CD (2015) Synthesis of reversed structured triacylglycerols possessing EPA and DHA at their terminal positions. Tetrahedron 71:8544–8550
Kawashima A, Shimada Y, Yamamoto M, Sugihara A, Nagao T, Komemushi S, Tominaga Y (2001) Enzymatic synthesis of high-purity structured lipids with caprylic acid at 1, 3-positions and polyunsaturated fatty acid at 2-position. J Am Oil Chem Soc 78:611–616
Zhao XY, Wang XD, Liu X, Zhu WJ, Mei YY, Li WW, Wang J (2015) Structured lipids enriched with unsaturated fatty acids produced by enzymatic acidolysis of silkworm pupae oil using oleic acid. Eur J Lipid Sci Technol 117:879–889
Zhao HZ, Lu ZX, Lu FX, Bie XM, Liu ZM, Zeng XX (2006) Lipase-catalysed acidolysis of lard with caprylic acid to produce structured lipid. Int J Food Sci Technol 41:1027–1032
Khodadadi M, Kermasha S (2014) Modeling lipase-catalyzed interesterification of flaxseed oil and tricaprylin for the synthesis of structured lipids. J Mol Catal B-Enzym 102:33–40
Nunes PA, Pires-Cabral P, Guillén M, Valero F, Ferreira-Dias S (2012) Optimized production of MLM triacylglycerols catalyzed by immobilized heterologous Rhizopus oryzae lipase. J Am Oil Chem Soc 89:1287–1295
Chen B, Zhang H, Cheong LZ, Tan T, Xu X (2012) Enzymatic production of ABA-type structured lipids containing omega-3 and medium-chain fatty acids: effects of different acyl donors on the acyl migration rate. Food Bioprocess Technol 5:541–547
Romdhane IBB, Fendri A, Gargouri Y, Gargouri A, Belghith H (2010) A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem Eng J 53:112–120
Romdhane IBB, Frikha F, Maalej-Achouri I, Gargouri A, Belghith H (2012) Gene cloning and molecular characterization of the Talaromyces thermophilus lipase catalyzed efficient hydrolysis and synthesis of esters. Gene 494:112–118
Romdhane IBB, Romdhane ZB, Bouzid M, Gargouri A, Belghith H (2013) Application of a chitosan-immobilized Talaromyces thermophilus lipase to a batch biodiesel production from waste frying oils. Appl Biochem Biote 171:1986–2002
Wang XM, Li DM, Qu M, Durrani R, Yang B, Wang YH (2017) Immobilized MAS1 lipase showed high esterification activity in the production of triacylglycerols with n-3 polyunsaturated fatty acids. Food Chem 216:260–267
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Li DM, Qin XL, Wang JR, Yang B, Wang WF, Huang WL, Wang YH (2015) Hydrolysis of soybean oil to produce diacylglycerol by a lipase from Rhizopus oryzae. J Mol Catal B-Enzym 115:43–50
Ota Y, Itabashi Y, Hasuo M (1996) Measurement of positional specificity index of microbial lipases by chiral phase high-pressure liquid chromatography. Biosci Biotechnol Biochem 60:145–146
Sahin N, Akoh CC, Karaali A (2005) Lipase-catalyzed acidolysis of tripalmitin with hazelnut oil fatty acids and stearic acid to produce human milk fat substitutes. J Agric Food Chem 53:5779–5783
Wang YH, Mai QY, Qin XL, Yang B, Wang ZL, Chen HT (2009) Establishment of an evaluation model for human milk fat substitutes. J Agric Food Chem 58:642–649
Li DM, Wang WF, Qin XL, Li XX, Yang B, Wang YH (2016) A novel process for the synthesis of highly pure n-3 polyunsaturated fatty acid (PUFA)-enriched triglycerides by combined transesterification and ethanolysis. J Agric Food Chem 64:6533–6538
Basso A, Froment L, Hesseler M, Serban S (2013) New highly robust divinyl benzene/acrylate polymer for immobilization of lipase CALB. Eur J Lipid Sci Technol 115:468–472
Cai CS, Gao YQ, Liu Y, Zhong NJ, Liu N (2016) Immobilization of Candida antarctica lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synthesis. Food Chem 212:205–212
Li DM, Wang WF, Liu PZ, Xu L, Faiza M, Yang B, Wang YH (2017) Immobilization of Candida antarctica lipase B onto ECR1030 resin and its application in the synthesis of n-3 PUFA-rich triacylglycerols. Eur J Lipid Sci Technol 119:1700266
Acknowledgements
This work was supported by the National Outstanding Youth Science Foundation of China (31725022), Molecular Enzyme and Engineering International Cooperation Base of South China University of Technology (2017A050503001), Special Program of Guangdong Province for Leader Project in Science and Technology Innovation: Development of New Partial Glycerin Lipase (2015TX01N207), and Science and Technology Planning project of Guangdong province (2016B090920082).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Lian, W., Wang, W., Tan, C.P. et al. Immobilized Talaromyces thermophilus lipase as an efficient catalyst for the production of LML-type structured lipids. Bioprocess Biosyst Eng 42, 321–329 (2019). https://doi.org/10.1007/s00449-018-2036-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-2036-7