Abstract
A low-cost and sustainable cellulosic ethanol production is vital for fermentation-based industrial applications. Reducing the expenses of cellulose-deconstruction enzymes is one of the significant challenges to economic cellulose-to-ethanol conversion. Here, we report the improved ethanol production from corn stover after dry biorefining using a natural β-glucosidase-producing strain Clavispora NRRL Y-50464 with a low cellulase dose of 5 mg protein/g glucan under separate enzymatic hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) conditions. Strain Clavispora NRRL Y-50464 exhibited a superior ethanol fermentation performance over Saccharomyces cerevisiae DQ1 under both conditions. It produced an ethanol titer of 38.1 g/L within 96 h at a conversion efficiency of 55.5% with 25% solids loading (w/w) via SSF without addition of extra β-glucosidase supplement. Improved performance of Y-50464 on a bioreactor with a helical stirring apparatus confirmed its advantage over the conventional bioreactors originally designed for liquid fermentations in cellulosic ethanol conversion by SSF. The results of this study suggested that the strain Clavispora NRRL Y-50464 has a potential as a candidate for lower-cost cellulosic ethanol production from lignocellulosic materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renewable biofuels including cellulosic ethanol are attractive alternatives as transportation fuels to reduce the use of fossil fuels that aid mitigation of greenhouse gas emissions. A significant advance has been made in the past decades toward commercialization of cellulosic ethanol production [1]. However, challenges remain for sustainable and economic operations in full commercial scales [2]. The extra cost of cellulase cocktails is a major issue, which is necessary to deconstruct the cellulose structure and release fermentable sugars for ethanol production. For conventional corn-based ethanol technology, the expense of a fermentation cocktail is less than 3% of the total operation cost. In contrast, the cellulase cocktails currently used in the cellulosic ethanol production cost more than 30% of the total operation [3, 4]. Reduction of the production cost, especially for the expenses of the digestive enzyme, is vital for a sustainable and efficient cellulosic ethanol production from lignocellulosic materials.
Since most cellulase complexes produced from bacteria and fungi have insufficient β-glucosidase activity, a supplement of external β-glucosidase is required during enzymatic hydrolysis for cellobiose-to-glucose degradation [5, 6]. To reduce the cost of cellulase, significant efforts had been taken to enable ethanologenic yeast to secrete or tether β-glucosidase on the cell surface through genetic engineering approaches [7, 8]. However, adequate β-glucosidase activity and the rapid enzymatic hydrolysis process require a massive amount of mutant yeast cells, which is impractical for commonly applied simultaneous saccharification and fermentation (SSF) processes [7].
A recently reported strain Clavispora NRRL Y-50464 is a natural β-glucosidase-producing yeast which produces at least three forms of β-glucosidases, BGL1, BGL2 and BGL3 [9]. It has been demonstrated to produce cellulosic ethanol from lignocellulosic materials without addition of extra β-glucosidase via SSF [10, 11]. However, the previously reported process was less efficient in application with a relatively higher cellulase dose at 34 FPU/g corncob residues or 15.3 FPU/g corn stover biomass using Celluclast 1.5 L, a commercial cellulase without β-glucosidase activity. The lower efficiency was partially attributed to the conventional bioreactor commonly used for liquid fermentation, which is inefficient for cellulosic ethanol production from slurry of cellulosic solids. The need of process engineering including a different design of a suitable bioreactor for cellulosic ethanol production using SSF is realized [11]. After preliminary evaluations, we found that it was promising to obtain a more efficient ethanol fermentation using this β-glucosidase-producing strain with a low cellulase dose. Here, we applied a bioreactor with a helical stirring apparatus that provides sufficient mixing power and mass transferring capability during enzymatic hydrolysis for higher levels of cellulosic solids loading [12]. This process is currently the most suitable and more efficient for cellulosic ethanol production using SSF. In this study, we characterized ethanol production from corn stover using the β-glucosidase-producing strain Clavispora NRRL Y-50464 with a lower cellulase input at 5 mg protein/g glucan. The evaluation was compared using an ethanologenic yeast Saccharomyces cerevisiae as a control under both separated hydrolysis and fermentation (SHF) and SSF conditions.
Materials and methods
Corn stover and enzymes
Corn stover was obtained from Bayan Nur League, Inner Mongolia Autonomous Region, China. The collected corn stover was milled using a beater pulverizer (SF-300, Ketai Milling Equipment, Shanghai, China) to pass through a mesh with a diameter of 10 mm. The milled corn stover was stored under dry conditions in plastic bags until use.
A commercial cellulase enzyme powder Youtell #7 and a liquid β-glucosidase enzyme Youtell #184 were provided by Hunan Youtell Biochemical Co. (Yueyang, Hunan, China). The enzyme activity was evaluated using an NREL protocol LAP-006 and a method as previously described [13, 14]. Youtell #7 had a cellulase activity of 63.0 FPU/g and a β-glucosidase activity of 99.9 CBU/g. Youtell #184 had a β-glucosidase activity of 5678 CBU/mL. The protein content was determined by the Bradford method with bovine serum albumin (BSA) as a protein standard for Youtell #7 and #184 as 46.7 mg/g and 40.0 mg/g, respectively [15].
Strains and culture medium
A β-glucosidase-producing yeast Clavispora NRRL Y-50464 was obtained from ARS Patent Culture Collection, Peoria, IL USA. Cell cultures were maintained and precultured on a medium containing 50 g glucose, 3.0 g yeast extract, and 5.0 g peptone per liter. The fermentation or SSF operation was carried out on a medium with the following nutrient supplementations: 3.0 g/L yeast extract, 5.0 g/L peptone, 2.0 g/L KH2PO4, 1.0 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.1 g/L NaCl, and 1.0 mL/L of a trace element solution. The trace element solution consisted of 0.25 g CuSO4·5H2O, 0.169 g MnSO4·H2O, 0.287 ZnSO4·7H2O, and 0.238 CoCl2·6H2O per liter [10].
A thermotolerant strain Saccharomyces cerevisiae DQ1, also known as CGMCC2528 from China General Microorganism Collection Center, was used as a control [16]. S. cerevisiae DQ1 is unable to metabolize cellobiose into ethanol. For this stain, a supplement of nutrients, including 2.0 g/L KH2PO4, 1.0 g/L (NH4)2SO4, 1.0 g/L MgSO4·7H2O, and 1.0 g/L yeast extract, was applied into the fermentation system for ethanol production [16].
Dry acid pretreatment and biodetoxification
Corn stover was pretreated using a dry acid pretreatment method as described previously [17, 18]. Briefly, the solid corn stover and the liquid dilute sulfuric acid (7.2%, w/w) were concurrently fed into the pretreatment reactor (pCF20-1.6, Keli Chemical Equipment, Yantai, China) at a ratio of 2:1. The mix was incubated at 175 °C for 5 min under mild helical agitation. The pretreated corn stover contained 38.0% of glucan and 4.4% of xylan as analyzed following a two-step dilute sulfuric acid hydrolysis procedure [19]. The solid content of the pretreated corn stover was about 50%. Then, the pretreated corn stover was neutralized to pH 5.0 with 20% (w/w) calcium hydroxide slurry and disk milled to remove the long cellulose fibers. It was further incubated in a 15-L bioreactor at 28 °C and aeration for 48 h to remove the inhibitory compounds generated during the dry acid pretreatment procedures as previously described [20, 21]. There was no wastewater generated during the process of pretreatment and biodetoxification treatment. But a substantial amount of monomer xylose was consumed by A. resinae ZN1 after the inhibitors were degraded [20].
Enzyme hydrolysis
The pretreated corn stover was enzymatically hydrolyzed at 25%, 30%, and 35% solids loading (w/w) separately in a helical stirring bioreactor at 50 °C for 48 h with Youtell #7 at a dose of 5 mg protein/g glucan (equal to 6.7 FPU/g glucan). The pH was maintained automatically at 4.8 with 20% (w/w) sodium hydroxide. No antibiotics were incorporated in any phase of this study. The slurry of the hydrolyzed corn stover was centrifuged at 10,000 rpm for 10 min to obtain a clear corn stover hydrolysate to be used for the following fermentation experiments. Such obtained corn stover hydrolysate (CSH) at 25% solids loading (25% CSH) contained 57.8 g/L glucose, 15.6 g/L xylose, 13.4 g/L cellobiose, and 1.2 g/L acetic acid. The 30% CSH contained 66.2 g/L glucose, 15.4 g/L xylose, 14.1 g/L cellobiose, and 2.5 g/L acetic acid. The 35% CSH contained 79.6 g/L glucose, 13.2 g/L xylose, 16.5 g/L cellobiose, and 4.0 g/L acetic acid.
Simultaneous saccharification and fermentation
The SSF operation was carried out in a 5-L bioreactor with a helical stirring apparatus as described previously [12]. The pretreated and biodetoxified corn stover and the bioreactor used during SSF were autoclaved at 121 °C for 20 min. The corn stover was first prehydrolyzed to a liquid state at 25% (w/w) solids loading with 5 mg protein/g glucan of Youtell #7 at 50 °C with agitation at 150 rpm for 12 h at pH 4.8. Then, an inoculation was made at a 10% ratio (v/v) using a seeding yeast cultivated overnight. The SSF was maintained at 37 °C and the pH adjusted at 5.5 by automatic regulation with 5 M NaOH. For the SSF without β-glucosidase addition, no extra β-glucosidase was supplemented to the system. However, for the SSF with β-glucosidase addition, extra β-glucosidase Youtell #184 was added to the system at the beginning of prehydrolysis with a dose of 91.1 CBU/g glucan. Samples were taken periodically and supernatants were obtained by centrifugation at 13,000 rpm for 5 min. Fermentation profiles were analyzed using HPLC as previously described [22].
Analysis
Glucose, xylose, cellobiose, acetic acid, and ethanol in a sample were analyzed on HPLC (LC-20AD, Shimadzu, Kyoto, Japan) equipped with a Bio-rad Aminex HPX-87H column (Bio-rad, Hercules, CA, USA) and RID-10A detector (Shimadzu, Kyoto, Japan). A solution of 5 mM H2SO4 was used as eluent with a flow rate of 0.6 mL/min.
The cellulose conversion efficiency (as ethanol yield) was calculated using the method described by Zhang and Bao specifically for the high solid SSF process [23]:
where [C1] stands for the ethanol concentration in the culture broth (g/L), W is the total water input of SSF (g), f the cellulose fraction of corn stover feedstock, [Biomass] the dry corn stover concentration at the beginning of SSF (g/g), m the total weight of SSF (g), 0.511 the conversion factor for glucose to ethanol based on stoichiometric biochemistry of yeast, and 1.111 the conversion factor for cellulose equivalent to glucose.
Results and discussion
Cellobiose assimilation of Clavispora NRRL Y-50464
The cellobiose assimilation capacity of Clavispora NRRL Y-50464 was evaluated in comparison with S. cerevisiae strain DQ1 using either glucose or cellobiose as a sole carbon source. On a medium using glucose as the sole carbon source, both strains were able to grow a cell mass, consume the sugar and produce ethanol. However, Clavispora NRRL Y-50464 showed a significantly faster rate of cell growth and glucose consumption and produced almost the same amount of ethanol compared with S. cerevisiae DQ1 (Fig. 1a). Under cellobiose as the sole carbon source conditions, S. cerevisiae showed a minimum cell growth likely from the trace amount of glucose that existed in the yeast extract (Fig. 1b). It did not produce any ethanol and the cellobiose remained intact in the medium unutilized. In contrast, Clavispora NRREL Y-50464 displayed normal cell growth and depleted cellobiose in no more than 12 h for ethanol conversion (Fig. 1b). According to our previous studies, a large amount of β-glucosidase activity (specific activity of 1.20 U/mg/mL) was observed in crude cell protein extracts by in vitro assay when the strain Y-50464 was grown on cellobiose [10]. Thus, Clavispora NRRL Y-50464 was capable of transforming cellobiose into ethanol directly, which has a potential in reducing the use of additional β-glucosidase, a significant component of cellulase, for lower-cost cellulosic ethanol production.
Comparison of ethanol fermentation profiles between S. cerevisiae DQ1 and Clavispora NRRL Y-50464 in response to different sugar conditions. a Fermentation using glucose as the sole carbon source; and b fermentation using cellobiose as the sole carbon source. Fermentation conditions: 37 °C for Clavispora NRRL Y-50464 and 30 °C for S. cerevisiae DQ1 in a shaking incubator with the agitation speed of 150 rpm
Ethanol production from corn stover hydrolysate
The corn stover hydrolysate (CSH) was prepared at 25%, 30%, and 35% solid loading (w/w) separately. Following a previously reported process economic analysis [3], a cellulase dose of 5 mg protein/g glucan was applied in this study. Since Youtell #7 was observed to possess a low β-glucosidase activity (99 CBU/g), the product inhibition of glucose on cellulase was more serious at higher solids loading of corn stover. As a result, cellobiose accumulation in the CSH increased with the increase in the solids loading. The final cellobiose concentration of the CSH samples was 13.4, 14.1, and 16.5 g/L for solids loading of 25%, 30% and 35%, respectively.
The cell growth of Clavispora NRRL Y-50464 was significantly higher than that of S. cerevisiae DQ1 as measured by OD600 for all three solids loading levels (Fig. 2a). Both strains showed a decreased trend of cell density with the increased levels of solids loading in CSH. Acetic acid was observed at 1.2, 2.5, and 4.0 g/L for the solids loading in CSH of 25%, 30%, and 35%, respectively. The decreased cell growth under higher levels of solids loading was possibly due to the higher acetic acid and phenolic acid concentrations accumulated in the CSH. Clavispora NRRL Y-50464 produced significantly higher levels of ethanol for all solids loading CSH compared with those fermented by S. cerevisiae DQ1 (Fig. 2b). The ethanol production increased with the increase of the solids loading levels at 26 g/L for 25% CSH and the with highest at 38 g/L for 35% CSH. The increased portion of ethanol production was attributed to the cellobiose in the CSH, since strain Y-50464 is able to directly convert cellobiose into ethanol. Clavispora NRRL Y-50464 showed about 80% of cellobiose consumption and, as anticipated, S. cerevisiae strain DQ1 did not utilize any cellobiose at all (Fig. 2c). For SHF at 25% (w/w) solids loading, the cellulose conversion efficiency was about 51% during the enzymatic hydrolysis step. The ethanol metabolic yield was 80% and 65% in the fermentation step for strain Y-50464 and strain DQ1, respectively. Thus, the net ethanol production was less than 41% for strain Y-50464 and 33% for strain DQ1 from cellulose in the corn stover. The lower efficiency of enzymatic hydrolysis was partially attributed to the lower input of cellulase at 5 mg protein/g glucan and the product inhibition in the presence of high glucose concentration. In addition, a residue amount of xylitol as a by-product was observed from cellobiose conversion by strain Y-50454 [11]. Thus, the cellobiose-to-ethanol pathway in Y-50464 also needs to be optimized in the future for more efficient cellobiose conversion.
Comparison of ethanol fermentation performance between S. cerevisiae DQ1 and Clavispora NRRL Y-50464 from corn stover hydrolysate prepared at different solids loading levels at 25%, 30% and 35% (w/w). a Cell growth response as measured by OD600; b ethanol production under the same conditions; and c cellobiose consumption ratio from the corn stover hydrolysate. Fermentation conditions: 37 °C, 150 rpm in a shaking incubator using 25%, 30%, and 35% CSH, respectively
Ethanol production from SSF
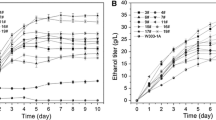
The SSF procedure is commonly applied for cellulosic ethanol production. It has an advantage in alleviating the glucose product inhibition on cellulase by converting the glucose into ethanol as it was released to reduce sugar accumulation. As a result, a higher ethanol yield often can be obtained relative to the operation using a separated hydrolysis and fermentation process [24]. In this study, we conducted comparative SSF for both strains applying 25% solids loading (w/w) of corn stover treated with 5 mg protein/g glucan using Youtell #7. For S. cerevisiae DQ1, ethanol production was lower over time without addition of β-glucosidase compared with that of added β-glucosidase treatment. In the absence of extra β-glucosidase, an ethanol titer of 30.0 g/L was obtained at 96 h after incubation. When β-glucosidase was added in the system, the ethanol titer was increased to 36.9 g/L for S. cerevisiae DQ1 (Fig. 3a; Table 1). This indicated an insufficient β-glucosidase activity of Youtell #7 applied, which resulted in the inefficient synergetic effect among the cellulase components (endo-, exo-glucanase, and β-glucosidase) and the poor cellulose conversion ratio. Unlike often observed cellobiose accumulation in CSH, no significant cellobiose accumulation occurred during SSF stage, even for the treatment without extra β-glucosidase supplementation. It reflected the advantage of SSF with a better alleviation of the product inhibition on cellulase.
Sugar conversion performance of simultaneous saccharification and fermentation from corn stover at 25% (w/w) solids loading. a Strain S. cerevisiae DQ1 performance with or without the addition of β-glucosidase in the medium; and b strain Clavispora NRRL Y-50564 performance with or without the addition of β-glucosidase in the medium. Conditions: prehydrolysis stage was maintained at 50 °C, pH 4.8 for 12 h, while the SSF stage was maintained at 37 °C, pH 5.5 for 84 h. The overnight precultured yeast seeds were inoculated at a ratio of 10% (v/v)
On the other hand, addition of extra β-glucosidase did not affect the performance of Clavispora NRRL Y-50464 at all. There was no significant difference in cellobiose consumption and ethanol conversion between SSF treatments with and without β-glucosidase supplementation (Fig. 3b). The fermentation treatment without addition of extra β-glucosidase produced an ethanol titer of 38.1 g/L with a conversion efficiency of 55.5% (Table 1). The treatment with added β-glucosidase generated a similar amount of ethanol with a titer of 37.7 g/L and conversion efficiency of 54.9%. Performance of both treatments by strain Y-50464 surpassed the fermentation by S. cerevisiae DQ1 under the same conditions as measured by the above parameters. These results suggested that the native β-glucosidase activity from strain Y-50464 was not only able to compensate the deficit of Youtell #7, but was also sufficient, by its native enzyme production, to complete the hydrolysis of cellobiose for ethanol conversion.
Ethanol production potential using strain Y-50464
Using a commercial cellulase Celluclast 1.5 L which lacks β-glucosidase activity, at a dose of 34 FPU/g, an ethanol production of 23 g/L was obtained previously from corncob residue without addition of β-glucosidase by SSF using Clavispora NRRL Y-50464 [10]. Using a similar method, only 17.2 g/L ethanol was obtained from conventional corn stover from NREL-DOE at 25% solids loading with an efficiency of 32.4% [11]. In this study, using a very low dose of 5 mg protein/g glucan from Youtell #7 (equal to 6.7 FPU/g glucan), we obtained a significantly higher ethanol production of 38.1 g/L from 25% solids loading. It also significantly improved the conversion efficiency up to 55.5%. Such a significant improvement is largely attributed to the bioreactor applied in this study, which has a special helical stirring apparatus that provides a strong mixing power and efficient mass transferring efficiency against the slurry of corn stover during the SSF process. Conversion of corn stover to ethanol was found to be more efficient at 15% solids loading using conventional bioreactors [11]. The efficiency decreased significantly with the increase in solids loading. For example, at 25% solids loading, the conversion efficiency only reached 32.4%. In this study, the conversion efficiency was 55.5% for 25% solids loading, which demonstrated the significant advantage of using the bioreactor with a unique helical stirring apparatus. Although the available sugar base from different corn stover sources varies and is difficult to compare, the large margin of the difference demonstrated by this study suggested that the helical stirring bioreactors used in this study were desirable for cellulosic ethanol production by SSF.
In addition to using adapted natural isolates, genetic engineering efforts have been made to enable S. cerevisiae-producing β-glucosidase. Ethanol production was evaluated from varied sources of cellulose including pure commercial cellulose and raw corn stover with different cellulase inputs (Table 2). In general, recombinant S. cerevisiae strains expressing cellobiose transporter or β-glucosidase did not produce satisfactory levels of cellulosic ethanol even with higher levels of cellulase input in the fermentation. It needs to be pointed out that we applied only 5 mg protein/g glucan of cellulase in this study to achieve a high ethanol production close to the minimum industrial standard of 40 g/L from genuine lignocellulosic materials such as corn stover using Clavispora NRRL Y-50464 without addition of extra β-glucosidase. Three forms of β-glucosidases, BGL1, BGL2 and BGL3, were characterized from Clavispora NRRL Y-50464 [9, 25]. Since additional enzyme activity was observed, other new forms of the enzyme are expected to be discovered in the future. These enzymes demonstrated hydrolysis activities toward at least 14 oligosaccharide substrates, including cellotetraose, cellopentaose, laminaritetraose, laminaripentaose, lactose, lichenan and other complex oligos with glycosidic bonds [9]. Such a comprehensive hydrolysis capability clearly contributed to a broad range of the β-glucosidase activity, which is a significant component of the cellulase complex for complete deconstruction of cellulosic materials for SSF.
The minimum industrial standard of ethanol production is 40 g/L. Clavispora NRRL Y-50464 produced 38.1 g/L without addition of extra β-glucosidase via SSF in this study, which came short than expected. Further improvement of the yeast performance is needed. For example, lignin was observed to inhibit cellulose-to-ethanol conversion and a delignified cellulose corn stover significantly improved ethanol production and conversion efficiency up to 65% [11]. Stain Y-50464 was able to produce 40.44 g/L ethanol from pure cellulose using low-efficient SSF with bottles [11]. This level of the titer is likely to be reached easily by application of the more efficient helical stirring bioreactors. In addition to converting cellobiose into ethanol, a small amount of xylitol as a by-product was also produced by strain Y-50464 at the end of SSF [10, 11]. Future improvement of the cellobiose-to-ethanol conversion pathway for Y-50464 is apparently needed to efficiently utilize available sugars for higher ethanol productivity. In addition, strain Y-50464 is suitable for ethanol production from cellulosic solids by SSF and limited in xylose-to-ethanol conversion. Thus, it may be more useful for specific niche applications, but not fit for conventional ethanol production from lignocellulosic hydrolysates which contain a large portion of xylose. With continued improvement of this β-glucosidase-producing yeast, it is expected to have applications in a more extended scale for cellulosic ethanol production.
Conclusions
The β-glucosidase-producing yeast strain Clavispora NRRL Y-50464 exhibited a superior ethanol fermentation performance over the control strain S. cerevisiae DQ1 in both SHF and SSF processes. An ethanol titer of 38.1 g/L and conversion efficiency of 55.5% were obtained from 25% solids loading (w/w) with a low cellulase dose of 5 mg protein/g glucan without addition of extra β-glucosidase. The strong native β-glucosidase activity generated from Clavispora NRRL Y-50464 provided sufficient complementary hydrolysis capability to reduce the enzyme cost for cellulosic ethanol production by SSF. This work also demonstrated that a bioreactor with a helical stirring apparatus is more suitable than the conventional bioreactor originally designed for liquid fermentation for improved cellulosic ethanol production by SSF. Clavispora NRRL Y-50464 demonstrated a potential as a candidate, with future improvement, for a lower-cost cellulosic ethanol production from lignocellulosic materials.
References
Lynd L, Liang X, Biddy M, Allee A, Cai H, Foust T, Himmel M, Laser M, Wang M, Wyman C (2017) Cellulosic ethanol: status and innovation. Curr Opin Biotechnol 45:202–211
Zhang Q, Bao J (2017) Industrial cellulase performance in the simultaneous saccharification and co-fermentation (SSCF) of corn stover for high-titer ethanol production. Bioresour Bioprocess 4:17
Liu G, Zhang J, Bao J (2016) Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst Eng 39:133–140
Valdivia M, Galan J, Laffarga J, Ramos J (2016) Biofuels 2020: biorefineries based on lignocellulosic materials. Microb Biotechnol 9:585–594
Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira N (2010) Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol Biofuels 3:3
Martins L, Kolling D, Camassola M, Dillon A, Ramos L (2008) Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol 99:1417–1424
Lee W, Nan H, Kim H, Jin Y (2013) Simultaneous saccharification and fermentation by engineered Saccharomyces cerevisiae without supplementing extracellular β-glucosidase. J Biotechnol 167:316–322
Haan R, Rensburg E, Rose S, Gorgens J, van Zyl W (2015) Progress and challenges in the engineering of non-cellulolytic microorganisms for consolidated bioprocessing. Curr Opin Biotechnol 33:32–38
Wang X, Liu ZL, Weber SA, Zhang X (2016) Two new native β-glucosidases from Clavispora NRRL Y-50464 confer its dual function as cellobiose fermenting ethanologenic yeast. PLoS One 11:e0151293
Liu ZL, Weber SA, Cotta M, Li S (2012) A new β-glucosidase producing yeast for lower-cost cellulosic ethanol production from xylose-extracted corncob residues by simultaneous saccharification and fermentation. Bioresour Technol 104:410–416
Liu ZL, Cotta M (2015) Technical assessment of cellulosic ethanol production using β-glucosidase producing yeast Clavispora NRRL Y-50464. Bioenerg Res 8:1203–1211
Zhang J, Chu D, Huang J, Yu Z, Dai G, Bao J (2010) Simultaneous saccharification and ethanol fermentation at high corn stover solids loading in a helical stirring bioreactor. Biotechnol Bioeng 105:718–728
Adney B, Baker J (1996) Measurement of cellulase activities. LAP-006. NREL analytical procedure. National Renewable Energy Laboratory, Golden CO
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 25:248–256
Chu D, Zhang J, Bao J (2012) Simultaneous saccharification and ethanol fermentation of corn stover at high temperature and high solids loading by a thermotolerant strain Saccharomyces cerevisiae DQ1. Bioenerg Res 5:1020–1026
Zhang J, Wang X, Chu D, He Y, Bao J (2011) Dry pretreatment of lignocellulose with extremely low steam and water usage for bioethanol production. Bioresour Technol 102:4480–4488
He Y, Zhang L, Zhang J, Bao J (2014) Helically agitated mixing in dry dilute acid pretreatment enhances the bioconversion of corn stover into ethanol. Biotechnol Biofuels 7:1
Sluiter A, Hames B, Ruiz R, Scarlat C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Technical Report NREL/TP-510-42618. Laboratory Analytical Procedure (LAP), Golden CO
Zhang J, Zhu Z, Wang N, Wang W, Bao J (2010) Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus Amorphotheca resinae ZN1 and the consequent ethanol fermentation. Biotechnol Biofuels 3:26
He Y, Zhang J, Bao J (2016) Acceleration of biodetoxification on dilute acid pretreated lignocellulose feedstock by aeration and the consequent ethanol fermentation evaluation. Biotechnol Biofuels 9:19
Zhang J, Lei C, Liu G, Bao Y, Balan V, Bao J (2017) In-situ vacuum distillation of ethanol helps to recycle cellulase and yeast during SSF of delignified corncob residues. ACS Sustain Chem Eng 5:11676–11685
Zhang J, Bao J (2012) A modified method for calculating practical ethanol yield at high lignocellulosic solids content and high ethanol titer. Bioresour Technol 116:74–79
Olofsson K, Bertilsson M, Liden G (2008) A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1:7
Liu ZL, Weber SA, Cotta M (2013) Isolation and characterization of a β-glucosidase from a Clavispora strain with potential applications in bioethanol production from cellulosic materials. Bioenerg Res 6:65–74
Shen Y, Zhang Y, Ma T, Bao X, Du F, Zhuang G, Qu Y (2008) Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresour Technol 99:5099–5103
Treebupachatsakul T, Nakazawa H, Shinbo H, Fujikawa H, Nagaiwa A, Ochiai N, Kawaguchi T, Nikaido M, Totani K, Shioya K, Shida Y, Morikawa Y, Ogasawara W, Okada H (2016) Heterologously expressed Aspergillus aculeatus β-glucosidase in Saccharomyces cerevisiae is a cost-effective alternative to commercial supplementation of β-glucosidase in industrial ethanol production using Trichoderma reesei cellulases. J Biosci Bioeng 121:27–35
Hu M, Zha J, He L, Lv Y, Shen M, Zhong C, Li B, Yuan Y (2016) Enhanced bioconversion of cellobiose by industrial Saccharomyces cerevisiae used for cellulose utilization. Front Microbiol 7:241
Chapla D, Parikh B, Liu L, Cotta M, Kumar A (2015) Enhanced cellulosic ethanol production from mild-alkali pretreated rice straw in SSF using Clavispora NRRL Y-50464. J Biobased Mater Bioenergy 9:1–8
Kumar AK, Parikh BS, Shah E, Liu ZL, Cotta MA (2016) Cellulosic ethanol production from green solvent-pretreated rice straw. Biocatal Agric Biotechnol 7:14–23
Acknowledgements
This research was supported by the Natural Science Foundation of China (21306048) and sponsored by Shanghai Pujiang Program. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Geberekidan, M., Zhang, J., Liu, Z.L. et al. Improved cellulosic ethanol production from corn stover with a low cellulase input using a β-glucosidase-producing yeast following a dry biorefining process. Bioprocess Biosyst Eng 42, 297–304 (2019). https://doi.org/10.1007/s00449-018-2034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-2034-9