Abstract
Aspergillus flavus: fungal strain A5p1 is reported here to decolorize a variety of dyes under broad environmental conditions. For the 15 dyes tested, the decolorization efficiencies ranged from 61.7 to 100.0% at an initial concentration of 100 mg/L. Direct Blue 71 (DB71), Direct Blue 86 (DB86), and Reactive Blue 19 (RB19) were selected as models for comparing decolorization performance. The results show that biosorption and biodegradation work together in the strain to remove the pigments; the effect of biosorption was stronger for DB71 and DB86, whereas the effect of biodegradation was stronger for RB19. For DB71 and DB86, the decolorization rate surpassed 90% with inactivated biomass under acidic conditions and with living biomass under alkaline conditions. DB19 achieved the highest removal rate of 90% under neutral conditions. The strain could effectively decolorize high concentrations of dyes up to 1000 mg/L, which was achieved mainly via biosorption at concentrations below 500 mg/L and via biodegradation at concentrations above 500 mg/L. The findings suggest that A5p1 has a strong adaptability to different dye types and environmental conditions and can, therefore, be potentially used in biological processes for the treatment of dye-containing wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past century, over 10,000 different synthetic dyes and pigments have been developed and applied for common use in industries such as textile dyeing, paper printing, plastics, and leather industries, with approximately 0.7 million tones of dyestuff manufactured each year [1]. Most of these dye components have exhibited carcinogenicity, teratogenicity, and mutagenesis for humans and other organisms; meanwhile, they are intentionally designed to be resistant to light, water, and oxidizing agents; they are difficult to remove once they are released into the environment [2]. Therefore, there is great interest in the rapid and cost-effective removal of dyes.
Biological processes of wastewater treatment are universally accepted to have technical and economic advantages over physical and chemical methods [3]. Many biological materials have demonstrated very good performance in removing pigments, including fungi such as white rot fungi [4,5,6]; bacteria such as Serratia liquefaciens [7], Bacillus sp. [8] and Sphingomonas [9]; and even algae such as Shewanella algae [10] and microalgae species [11]. These biological resources lay the foundation for the construction of dye biodecolorization processes.
Biosorption and biodegradation are two major mechanisms in the biodecolorization of dye wastewater. The former describes the process mediated by inactivated biomass [12, 13], and these materials are often referred to as being biosorbent. In living cells, the two mechanisms can act together [14, 15]. Given the wide variety of enzymes in a strain and the low selectivity of biosorbents, it is expected that the same strain can decolorize various dyes via different mechanisms, as demonstrated by many examples. The white rot fungus Coriolopsis sp. was tested to decolorize four pigments of different colors or common backbones [16], and Phanerochaete chrysosporium showed the decolorization ability for Acid Blue 62 [17], Direct Red 80 [18], and indigo dye [19]. In the case of Aspergillus flavus, there are reports of dye decolorization of RBBR [1], Navilan yellow RL [20], and Drimarene blue K(2)RL [21]. Less research has been devoted to the decolorization of various dyes by Aspergillus flavus alone.
Wastewater, including wastewater discharged from a single plant, inevitably contains complex pigments, because different types of pigment are employed based on the dyeing colors and textile properties. Therefore, a strain with a broad-spectrum substrate should have potential for use in an industrial process from the point of industrial application. In addition, it is easier to achieve high stability, flexibility, and adaptability in practice with a strain that is adaptable to a wide pH range and is tolerant to high dye concentrations. Here, we report a strain of Aspergillus flavus, named A5p1, which exhibited a good decolorizing effect on 15 pigments representing azo-based, anthraquinone-based, triphenylmethane-based, and phthalocyanine-based dyes. The decolorization mechanism was preliminarily examined and was found to change with dye type and external conditions. The results suggest that this strain has considerable potential for use in biodecolorization applications.
Materials and methods
Microorganism and culture medium
Aspergillus flavus A5p1 was isolated and preserved in theChina General Microbiological Culture Collection Center (CGMCC No. 4292). The medium consisted of the following (g L−1 of distilled water, pH adjusted to 5): D-glucose, 2.5; NaNO3, 2; KH2PO4, 1; MgSO4·7H2O, 0.5. Once inoculated, 250 mL Erlenmeyer flasks containing 60 mL of sterile medium were incubated in a controlled incubator at 150 rpm for 4 days at 39 °C.
Chemicals
All the reagents were of analytical grade and were mainly purchased from Macklin (Shanghai, China) and Aladdin (Shanghai, China). The dyes used in the study were obtained from Aladdin (Shanghai, China) and YuHua (Tainjin, China).
Biosorbents
The pellets were harvested after cultivation and washed several times with distilled water before being inactivated at 121 °C for 20 min. The dried mycelia were stored in the refrigerator and used for biosorption.
Crude enzyme liquid preparation
To grow the fungal biomass, 4.3 × 104 spores mL−1 of fungal suspension on a Potato Dextrose Agar medium (PDA) was inoculated in a 250 mL Erlenmeyer flask containing a 60 mL autoclaved solution of culture medium with dye of the desired concentration. The flasks were agitated at 150 rpm and at 39 °C for 4 days. The pellets were separated from the fermentation broth by centrifugation and homogenized in 0.05 mol/L, pH 7.0, phosphate buffer. The intracellular enzyme was harvested by centrifugation, and the extracellular enzyme was harvested by ultrafiltration of the cell culture filtrate. The crude enzyme liquid was obtained using a mixture of intracellular enzyme and extracellular enzyme.
Decolorizing experiments with living cells
To grow fungal biomass, 4.3 × 104 spores mL−1 of the fungal suspension on a PDA plate was inoculated in an Erlenmeyer flask containing a 60 mL autoclaved solution of culture medium with dye of the desired concentration. The flasks were agitated at 150 rpm at 39 °C. Sample solution (1 mL) was collected from the flasks after 8 days and separated by centrifugation (Allegra 25R, Beckman, USA) at 8000 rpm for 10 min; the supernatant fraction was analyzed for the remaining dye ions. The results, presented as averages, were obtained from experiments performed at least three times.
To evaluate the influences of pH and dye concentration, one experiment was performed at pH 3 to 11 with 500 mg/L CDB71, 500 mg/L CDB86, and 300 mg/L CRB19, and another assayed each dye at concentrations ranging from 100 to 2000 mg/L at pH 5.0. The mixed-dye decolorization experiments were performed at pH 5.0 with total concentrations of 0.3 g/L (0.1 g/L CDB71, 0.1 g/L CDB86, 0.1 g/L CRB19), 0.5 g/L (0.2 g/L CDB71, 0.2 g/L CDB86, 0.1 g/L CRB19), and 0.6 g/L (0.2 g/L CDB71, 0.3 g/L CDB86, 0.1 g/L CRB19).
Decolorizing experiments using inactivated cells
Culture medium (60 mL) with dye of the desired concentration and inactivated fungal biomass (approximately 0.3 g, i.e., the dry weight of living pellets in culture medium with dye on the eighth day) was shaken in a controlled incubator at 150 rpm at 39 °C. Sample solution (1 mL) was collected from the flasks after 1 day and separated by centrifugation (Allegra 25R, Beckman, USA) at 8000 rpm for 10 min; the supernatant fraction was analyzed for residual dye concentration. The results, presented as averages, were obtained from experiments performed at least three times. To evaluate the influence of pH and dye concentration, an experiment was performed at pH 3 to 11 with 500 mg/L CDB71, 500 mg/L CDB86, and 300 mg/L CRB19, and each dye was assayed at concentrations ranging from 100 to 2000 mg/L at pH 5.0.
Decolorizing experiments by crude enzyme liquid
Culture medium (60 mL) with dye concentrations of 200 mg/L CDB71, 300 mg/L CDB86, and 100 mg/L CRB19 and 1 mL crude enzyme was shaken in a controlled incubator at 150 rpm and at 39 °C. Sample solution (1 mL) was collected from the flasks at defined time points and separated by centrifugation (Allegra 25R, Beckman) at 8000 rpm for 10 min. The supernatant fraction was analyzed for the remaining dye ions. The results, provided as averages, were obtained from experiments performed three times.
Analysis
Manganese peroxidase (MnP) (EC 1.11.1.13) activity was based on the color change due to oxidation of 2; 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid (ABTS)). The increase in absorbance was measured at 420 nm at 24 °C using a ε420 value of 36,000 M−1 cm−1 to calculate MnP activity [22].
Glucose oxidase (GOD) (EC 1.1.3.4) activity was determined by measuring H2O2 production. The H2O2 produced was utilized to oxidize a chromomeric substrate in the presence of horseradish peroxidase [23].
The absorbance of each sample was analyzed using a UV/Vis spectrophotometer (Tu-1900, PERSEE, China).
The decolorization percentage was calculated with the following formula:
where A0 is the initial absorbance and A is the final absorbance.
Results and discussion
Broad-spectrum dye uptake ability of Aspergillus flavus A5p1
According to their chemical structure, dyes are generally classified as follows: azo-based, anthraquinone-based, phthalocyanine-based, and triphenylmethane-based dyes [24]. Fifteen dyes representing the above four types were chosen to test the decolorization ability of A. flavus A5p1. The results shown in Table 1 demonstrate that the strain had different decolorization effects over the 15 dyes. A decolorization rate of up to 100.0% was achieved for Direct Blue 71, even at 500 mg/L, and a moderate level of 64.0% was achieved for Acid Orange II at 100 mg/L. These results suggest that A. flavus A5p1 has a broad-spectrum degradation ability for dyes. Few studies have reported dye decolorization by Aspergillus flavus, and these studies focused on a single pigment. For example, Aspergillus flavus showed 85.0% decolorization for Reactive Red 198 at a concentration of 50 ppm [25], and Bruno Perlatti et al. reported the ability of Aspergillus flavus to decolorize Remazol Brilliant Blue R [1].
The decolorization efficiency of a strain is related to the chemical structure of the dye [26]. In our case, anthraquinone and triphenylmethane dyes appeared to be more resistant to degradation, especially compared to azo and phthalocyanine dyes, likely due to the aromatic structure of the former dyes. A decreased decolorization rate was observed with a decrease in dye molecular weight for azo dyes, from 99.9% for Direct Blue 71 to 31.1% for Acid Orange II. The presence of more sulfonic groups in the large azo (Fig. s1) might partially explain this effect, since one mechanism for color removal is based on the electrostatic interaction between negative sulfonic groups and the positive ammonium group on the cell surface [27].
Decolorization mechanisms
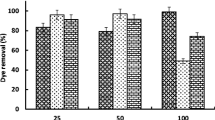
Three dyes, including Direct Blue 71 (DB71), Direct Blue 86 (DB86), and Reactive Blue 19 (RB19), were selected in this study to compare decolorization mechanisms. To evaluate the effect of biosorption and biodegradation, the decolorization of dyes was performed separately by inactivated and living biomass. As shown in Fig. 1a, a decolorization rate of 83.4, 100, and 49.4% was obtained for DB71, DB86, and RB19, respectively, using inactivated cells. These values suggested that the A5p1 biomass played a role in adsorption with respect to the tested dye molecules, since the mechanism for inactivated biomass is based principally on adsorption. For living biomass, cell growth and dye decolorization were synchronous over the entire incubation period (Fig. 1b). A comparison of the decolorization rate between the two types of biomass revealed that decolorization of RB19 by living biomass was approximately twice that of the inactivated biomass, but no significant differences were seen for DB71 and DB86. As degradation and adsorption are typically of the same importance and effect for decolorization mediated by living biomass, the role of degradation cannot be ignored for RB19, as the decolorization by living biomass was approximately twice that by inactivated biomass.
To further illustrate the function of biodegradation in decolorization, crude enzyme solution in the presence and in absent of dyes were prepared from the culture supernatant and mycelium. The results in Fig. 2 show that the enzyme in the presence of dye had better decolorization capability than the absence of dye, with rates of 100, 100.0, and 71.8% for DB71, DB86, and RB19, respectively; this indicates that the activity of the crude enzyme solution may be increased due to the presence of the dye. We also compared the growth curves of A5p1 in the presence or absence of dye and found that the presence of dye did not significantly increase cell growth (Fig. 1b). Moreover, the activities of two key enzymes, glucose oxidase (GOD) (EC 1.1.3.4) and manganese peroxidase (MnP) (EC 1.11.1.13), are commonly thought to be involved in the removal process [4, 28]. These enzymes were measured during culture in medium with and without dye. Both enzymes were detected in all cases, but their activities were much higher in media with dye than in media without (Table 2). MnP activities increased 3.2-fold in the presence of DB86, and GOD activities were 138-fold higher without DB71. The above finding indicated that the presence of dye might act as an activator to stimulate the secretion of key enzymes by A5p1. As these enzymes act as the machinery for degradation [29, 30], we assume that biodegradation plays a role in the decolorization of the three studied dyes but might be slower than adsorption for DB71 and DB86. Thus, strain A. flavus A5p1 might be flexible in the mechanism underlying the decolorization of different dyes.
Effect of pH on dye uptake
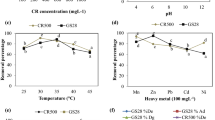
The effect of pH on the decolorization of the three dyes was performed separately for inactivated and living biomass. Figure 3a, b shows the large difference between the two biomass types. For inactivated biomass, DB71 and DB86 followed a similar tendency at a pH of 3.0 to 11.0. Decolorization was more efficient under acidic conditions than under basic conditions and declined sharply with increasing pH (Fig. 3a). This result can easily be explained by the interaction between negatively charged dye anions and positively charged cell surfaces, as reported for the Congo Red and coir pith carbon [31, 32]. In contrast, the removal capacity of RB19 was little affected by pH and remained at a low level, possibly due to the presence of fewer dissociated functional groups in RB19 [33].
For living biomass, the decolorization rate of RB19 and DB86 showed a bell curve with increasing pH, as shown in Fig. 3b. The maximum, above 90%, was observed under neutral conditions, and acidity or alkalinity lowered the removal rate, especially for RB19. This finding is consistent with the basic characteristics of enzymatic reactions, i.e., pH has significant effects on enzyme production and activity. The decolorization rate for DB71 was less affected by pH than was that for the other two dyes and maintained a high level over a wide pH range. These findings suggest that A. flavus A5p1 might decolorize via different mechanisms for different dyes and in different environments. For RB19, as speculated in the previous section, the decolorization mechanism mainly involved biodegradation by key enzymes in A. flavus A5p1. Even in the alkaline environment, where enzyme activity was decreased, the removal rate by living biomass was higher than that by inactivated biomass. For the other two dyes, DB71 and DB86, acidic conditions benefited adsorption, and a slightly higher removal rate was achieved for inactivated biomass than for living biomass. In an alkaline environment, biodegradation played a key role, allowing a high removal rate to be achieved despite a sharp decrease in adsorption rate. We view these behaviors as favorable; the mechanism of A.flavus A5p1 can adjust not only to the type of dye but also the external environment. This flexibility is desirable for the treatment of dye wastewater of complex composition under changing conditions.
Effect of initial dye concentration on dye uptake
Figure 4 shows the different effects of initial dye concentration on the removal of three types of dyes using inactivated and living biomass separately. A high decolorization rate for DB71 and DB86 at concentrations below 500 mg/L was achieved with whichever kind of cell, living cell, or inactivated biomass, which reinforces the critical role of biosorption in decolorization from the other side. Figure 4 also shows that the absorptive capacity for these two dyes was reached at 1000 mg/L, which is consistent with the higher decolorization attained with living biomass than with inactivated biomass at concentrations above 500 mg/L. For RB19, the absorptive capacity was far lower than that for the other two dyes, and saturation was reached at 250 mg/mL. Accordingly, the decolorization rate was higher for living biomass than for inactivated biomass over the entire concentration range. These findings are in agreement with those discussed in the previous section and demonstrate that the mechanism of A. flavus A5p1 can adjust to the external environment.
When active biomass is employed to treat wastewater with a high concentration of dye, strain growth is easily inhibited by the dye toxicity. A summary of the literature in which fungi are employed for the treatment of dyes is provided in Table 3. Compared with previously studied strains, which were generally effective at low concentrations, strain A. flavus A5p1 worked well at high dye concentrations. The decolorization rate reached 75.8 and 89.8% at 1000 mg/L of DB71 and DB86, respectively, and it reached 72.5% at 500 mg/L of RB19. The above results indicate that A. flavus A5p1 has obvious advantages for decolorizing highly concentrated dye wastewater and is suitable for meeting the requirements of actual wastewater treatment.
Decolorization of mixed-dye solution
To understand the ability of a strain to treat actual wastewater, some researchers have studied the removal of mixed-dye effluent; examples include the study of eight Trametes versicolor-degraded azo dyes [38] and the biodecolorization of Direct Red-80 (DR-80) and Mordant Blue-9 (MB-9) by Phanerochaete chrysosporium [39]. Since strain A. flavus A5p1 showed good decolorization ability for a single dye, we tested its ability to decolorize a mixture of dyes. The results in Fig. 5 show that A. flavus A5p1 yielded a decolorization rate of 98.8, 90.5, and 78.5% at initial mixed-dye concentrations of 0.3, 0.5 and 0.6 g/L, respectively, indicating high efficacy. Due to the complexity of the mixture system, future work might include removal kinetics, reactor design, and operation and other factors. Such efforts may enable A. flavus A5p1 to be used in a decoloring technique for real wastewater.
Decolorization of mixed-dye wastewater by live A5p1 at different initial dye concentrations (CMix dye 0.3 g/L = CDB71 0.1 g/L + CDB86 0.1 g/L + CRB19 0.1 g/L; CMix dye 0.5 g/L = CDB71 0.2 g/L + CDB86 0.2 g/L + CRB19 0.1 g/L; CMix dye 0.6 g/L = CDB71 0.2 g/L + CDB86 0.3 g/L + CRB19 0.1 g/L; pH 5.0; T = 39 °C)
Conclusions
The present study demonstrated the high potential of strain A5p1 of Aspergillus flavus for decolorization of a wide range of dye substrates. The decolorization mechanism was very flexible, enabling the strain to adapt to a variety of environments involving different pH conditions and dye concentrations and attain decolorization via both adsorption and biodegradation. The studied strain should be viewed as a promising candidate for the biotechnological treatment of industrial color effluents.
References
Perlatti B, Fernandes JB, Forim MR (2012) Validation and application of HPLC-ESI-MS/MS method for the quantification of RBBR decolorization, a model for highly toxic molecules, using several fungi strains. Biores Technol 124:37–44
Siddique M, Farooq R, Price GJ (2014) Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason Sonochem 21:1206–1212
Hayat K, Menhas S, Bundschuh J et al (2017) Microbial biotechnology as an emerging industrial wastewater treatment process for arsenic mitigation: a critical review. J Clean Prod 151:427–438
Ansari Z, Karimi A, Sedghi S et al (2017) Glucose oxidase effect on treatment of textile effluent containing reactive azo dyes by Phanerochaete chrysosporium. J Chem Technol Biotechnol 92:1721–1726
Junghanns C, Neumann JF, Schlosser D (2012) Application of the aquatic fungus Phoma sp. (DSM22425) in bioreactors for the treatment of textile dye model effluents. J Chem Technol Biotechnol 87:1276–1283
He F, Qin X, Zhang H et al (2015) Characterization of laccase isoenzymes from the white-rot fungus Ganoderma sp.En3 and synergistic action of isoenzymes for dye decolorization. J Chem Technol Biotechnol 90:2265–2279
Haq I, Kumar S, Kumari V et al (2016) Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. J Hazard Mater 305:190–199
Mahmood F, Shahid M, Hussain S et al (2017) Potential plant growth-promoting strain Bacillus sp. SR-2–1/1 decolorized azo dyes through NADH-ubiquinone:oxidoreductase activity. Biores Technol 235:176–184
Ding J, Zhang Y, Xie Q et al (2015) Anaerobic biodecolorization of AO7 by a newly isolated Fe (III)-reducing bacterium Sphingomonas strain DJ. J Chem Technol Biotechnol 90:158–165
Meng X, Liu G, Zhou J et al (2014) Effects of redox mediators on azo dye decolorization by Shewanella algae, under saline conditions. Biores Technol 151:63–68
Baldev E, Mubarakali D, Ilavarasi A et al (2013) Degradation of synthetic dye, Rhodamine B to environmentally non-toxic products using microalgae. Colloids Surf B Biointerfaces 105:207–214
Akar T, Demir TA, Kiran I et al (2010) Biosorption potential of Neurospora crassa cells for decolorization of Acid Red 57 (AR57) dye. J Chem Technol Biotechnol 81:1100–1106
Li R, Ning XA, Sun J et al (2015) Decolorization and biodegradation of the Congo red by Acinetobacter baumannii YNWH 226, and its polymer production’s flocculation and dewatering potential. Biores Technol 194:233–239
Reddy A (1995) The potential for white-rot fungi in the treatment of pollutants. Curr Opin Biotechnol 6:320–328
Teerapatsakul C, Parra R, Keshavarz T et al (2017) Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int Biodeterior Biodegradation 120:52–57
Chen SH, Yien Ting AS (2015) Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost. J Environ Manage 150:274–280
Faraco V, Pezzella C, Giardina P et al (2009) Decolourization of textile dyes by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus. J Chem Technol Biotechnol 84:414–419
Singh S, Pakshirajan K, Daverey A (2010) Enhanced decolourization of Direct Red-80 dye by the white rot fungus Phanerochaete chrysosporium employing sequential design of experiments. Biodegradation 21:501–511
Miranda RD, Gomes CMD, Pereira N, et al (2013) Biotreatment of textile effluent in static bioreactor by Curvularia lunata, URM 6179 and Phanerochaete chrysosporium, URM 6181. Biores Technol 142:361–367
Ghosh A, Dastidar MG, Sreekrishnan TR (2016) Bioremediation of a Chromium Complex Dye Using Aspergillus flavus and Aspergillus tamari. Chem Eng Technol 39:1636–1644
Andleeb S, Atiq N, Robson GD et al (2012) An investigation of anthraquinone dye biodegradation by immobilized Aspergillus flavus in fluidized bed bioreactor. Environ Sci Pollut Res 19:1728–1737
Hofrichter M, Vares K, Scheibner K et al (1999) Mineralization and solubilization of synthetic lignin by manganese peroxidases from Nematoloma frowardii, and Phlebia radiate. J Biotechnol 67:217–228
Bankar SB, Bule MV, Singhal RS et al (2009) Glucose oxidase—an overview. Biotechnol Adv 27:489–501
Fleischmann C, Lievenbrück M, Ritter H (2015) Polymers and dyes: developments and applications. Polymer 7:717–746
Esmaeili A, Kalantari M (2012) Bioremoval of an azo textile dye, Reactive Red 198, by Aspergillus flavus. World J Microbiol Biotechnol 28:1125–1131
Chen Y, Chen G, Chen L et al (2011) Review of Studies on Effects of Molecular Structure on Azo Dye Microbial Decolorization. Environ Sci Technol 34:65–69
Lin-Na D, Bing W, Gang L, Sheng W, David EC, Yu-Hua Z (2012) Biosorption of the metal-complex dye Acid Black 172 by live and heated biomass of Pseudomonas sp. Strain DYl: kinetics and sorption mechanisms. J Hazard Mater 205:47–54
Espinosa-Ortiz EJ, Rene ER, Pakshirajan K et al (2016) Fungal pelleted reactors in wastewater treatment: applications and perspectives. Chem Eng J 283:553–571
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Lan J, Huang X, Hu M et al (2006) High efficient degradation of dyes with lignin peroxidase coupled with glucose oxidase. J Biotechnol 123:483–490
Binupriya AR, Sathishkumar M, Swaminathan K et al (2008) Comparative studies on removal of Congo red by native and modified mycelial pellets of Trametes versicolor in various reactor modes. Biores Technol 99:1080–1088
Namasivayam C, Kavitha D (2002) Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigment 54(1):47–58
Aksu Z, Tatlı Aİ, Tunç Ö (2008) A comparative adsorption/biosorption study of Acid Blue 161: effect of temperature on equilibrium and kinetic parameters. Chem Eng J 142:23–39
Permpornsakul P, Prasongsuk S, Lotrakul P et al (2016) Treatment of an azo dye reactive black 5 by tropical resupinate fungus Phanerochaete sordida, PBU 0057. New Biotechnol 33:135–136
Jin R, Hua Y, Zhang A et al (2009) Bioaugmentation on decolorization of C.I. Direct Blue 71 by using genetically engineered strain Escherichia coli, JM109 (pGEX-AZR. J Hazard Mater 163:1123–1128
Jasińska A, Paraszkiewicz K, Sip A et al (2015) Malachite green decolorization by the filamentous fungus Myrothecium roridum, mechanistic study and process optimization. Biores Technol 194:43–48
Paz A, Carballo J, Pérez MJ et al (2017) Biological treatment of model dyes and textile wastewaters. Chemosphere 181:168
Martins MAM, Lima N, Silvestre AJD et al (2003) Comparative studies of fungal degradation of single or mixed bioaccessible reactive azo dyes. Chemosphere 529:67–73
Pakshirajan K, Singh S (2010) Decolorization of synthetic wastewater containing azo dyes in a batch-operated rotating biological contactor reactor with the immobilized fungus Phanerochaete chrysosporium. Ind Eng Chem Res 49:7484–7487
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21066001 and 51108098). The authors are grateful to American Journal Experts for English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ning, C., Qingyun, L., Aixing, T. et al. Decolorization of a variety of dyes by Aspergillus flavus A5p1. Bioprocess Biosyst Eng 41, 511–518 (2018). https://doi.org/10.1007/s00449-017-1885-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1885-9