Abstract
The moving bed biofilm reactor (MBBR), operated as a post carbon removal system, requires long start-up times in comparison to carbon removal systems due to slow growing autotrophic organisms. This study investigates the use of carriers seeded in a carbon rich treatment system prior to inoculation in a nitrifying MBBR system to promote the rapid development of nitrifying biofilm in an MBBR system at temperatures between 6 and 8 °C. Results show that nitrification was initiated by the carbon removal carriers after 22 h of operation. High throughput 16S-rDNA sequencing indicates that the sloughing period was a result of heterotrophic organism detachment and the recovery and stabilization period included a growth of Nitrosomonas and Nitrospira as the dominant ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB) in the biofilm. Peripheral microorganisms such as Myxococcales, a rapid EPS producer, appear to have contributed to the recovery and stabilization of the biofilm.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high occurrence of ammonia as a deleterious substance in the aquatic environment has created a worldwide demand for cost-effective and compact technologies to upgrade current wastewater treatment systems for ammonia removal [1]. The most efficient and conventional approach for ammonia removal is nitrification [2], which can be performed in a suspended growth or biofilm treatment system. Biofilm processes allow for nitrification with decreased sludge production and have demonstrated an ability to withstand acute toxicity [3, 4]. Specifically, the moving bed biofilm reactor (MBBR) has shown potential as a nitrifying upgrade option at temperate climates and recently at cold climates in the northern United States, Canada and Europe [5–7].
The MBBR process uses carriers, which remain in suspension and are circulated through the entire reactor volume by mixing or aeration. These carriers provide a surface for biofilm attachment and proliferation. One of the challenges with regard to nitrifying MBBR systems is the start-up period and in particular the time required to generate stable nitrifying biofilms. Both groups of bacteria responsible for the process of nitrification, ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB) are autotrophic and are very slow growing with limited abilities to produce extracellular polymeric substance (EPS) [8, 9]. Practical experiences in the start-up of post carbon removal nitrifying MBBR systems demonstrate start-up times in North America of 6 to 8 weeks at warm temperatures.
Efforts have been made to decrease the time period required for permanent attachment of nitrifying biomass and development of nitrifying biofilm. Methods investigated have included cellular entrapment as well as immobilization on surface-modified membranes, both demonstrating varying degrees of success [8, 10, 11]. These techniques, however, did not investigate the start-up at low temperatures often experienced in the northern climate regions. With the growing demand for nitrification and the use of mobile treatment units in remote locations, rapid start-up is highly beneficial to ensure process performance.
A recent hybrid technology for secondary treatment is the integrated fixed film activated sludge (IFAS) process which incorporates MBBR carriers in a conventional activated sludge (CAS) aeration tank. MBBR carriers are inserted into the aeration tank of a CAS to create the hybrid suspended and attached growth IFAS treatment system. Municipal, secondary treatment IFAS systems have become increasingly installed in North America and Europe as zero footprint upgrade technologies for CAS. These IFAS systems are typically operated at carbon (cBOD5) to nitrogen (total Kjeldahl nitrogen) (C/N) ratios of 4–6 [12]. Under conventional, secondary IFAS treatment conditions that are not specifically designed for nitrification, a heterotrophic biofilm is generated on the added carriers that often include AOB and NOB populations embedded in the biofilm; with the nitrifying populations representing less than 1% relative abundance of the total bacterial population [13]. As such, seeded carriers from secondary IFAS treatment systems (the most common application of IFAS in North America and hence the most readily available source of seeded carriers) may be suitable sources of inoculated carriers to promote the rapid start-up of a post carbon removal nitrifying MBBR system. However, the kinetic benefits of using seeded carbon-removal IFAS carriers to inoculate nitrifying MBBR systems remains unknown and further it is unclear how the biofilm and nitrifying biomass of the IFAS carriers will respond to a sudden shift from an organic carbon rich environment to a post carbon removal, ammonia rich environment.
The overall objective of this study is to investigate post carbon removal nitrification MBBR start-up at low temperatures seeded with carbon removal IFAS media. The study explores new information at the macro, meso and micro-scale for low temperature start-up. The specific objectives are to determine the start-up time and kinetic efficiency at low temperature with seeded IFAS media; to quantify the biofilm response as it relates to biofilm thickness, biofilm mass, biofilm density and production of total suspended solids (TSS); to characterize the bacterial communities in the IFAS biofilm and the microbial community shift of the MBBR biofilm during the start-up phase using next generation high-throughput Illumina sequencing.
Materials and methods
MBBR pilot system
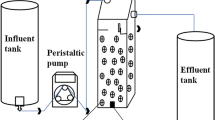
The pilot MBBR system consisted of two parallel aerobic MBBR treatment reactors, denoted MBBR1 and MBBR2. The pilot was installed as a means of post carbon removal treatment for ammonia removal at the Masson Angers lagoon treatment facility. The effluent from the lagoon (and hence the influent to the pilot) SCOD and ammonia concentrations were stable at 30 mg/L and 22 mg-N/L, respectively. The temperature, pH and dissolved oxygen (DO) in both reactors were constant at 7.6 ± 0.7 °C, 7.0 ± 0.1 and 8.8 ± 0.1 mg/L, respectively.
The pilot reactors were operated at low loading rates (LRs) throughout the start-up. MBBR systems are typically loaded at approximately half of the design LR during start-up to promote biofilm development. Operating at half of typical nitrifying design LRs also decreases the shock effect to the seeded carriers, which may promote complete failure of the biofilm matrix [14]. As conventional LRs for post carbon removal nitrifying MBBR systems are approximately 240–360 gN/m3 d, the start-up LRs for the two treatment reactors in this study were set to 144 and 86 gN/m3 d with each reactor having an HRT of 5.6 h (Table S-1). The LRs were altered based on carrier volume with MBBR2 having 1.6 times more carriers than MBBR1.
Biofilm samples
The carriers used to seed the MBBR system in this study were harvested from a secondary, carbon removal IFAS treatment plant located in Hawkesbury, Ontario, Canada. The carriers harvested from the IFAS system is AnoxKaldnes K5 with a surface area/volume of 800 m2/m3. The IFAS system was operated at carbon and nitrogen loads of 3200 gCOD/m3 d and 220 gN/m3 d, respectively. The average influent temperature, soluble chemical oxygen demand (SCOD) and ammonia (NH4-N/NH3-N) concentrations were 9.0 ± 0.1 °C, 230 ± 2 mg/L and 23.9 ± 0.6 mg/L, respectively. Using conventional biochemical oxygen demand to SCOD ratio of 0.5 [2], the biofilm in the IFAS system was acclimatized to a C/N ratio of 4.8. The hydraulic retention time (HRT) of the treatment unit was 2.7 h and the removal efficiencies for SCOD and ammonia were 57 and 27%, respectively. The IFAS system was in operation for over 2 years; hence, the biofilm on the harvested carriers were considered fully acclimatized to the IFAS system. Upon harvesting of the carriers, the carriers were immediately transported to the MBBR pilot system, located 45 min away in Masson Angers, Quebec, Canada.
Constituent analysis
Nitrification kinetic rates were measured twice per week in triplicate throughout the experimental period. Ammonia (NH4/NH3-N), nitrite (NO2-N) and nitrate (NO3-N) concentrations along with TSS and volatile suspended solids (VSS) were measured using standard methods [15]. SCOD concentrations were measured using HACH method 8000. Biofilm mass was measured using the protocol adopted from Delatolla et al. [16]. Specifically, the carriers with attached biofilm were dried at 105 °C for 24 h and the mass of the carrier with biofilm was recorded (M1). The biofilm from the carriers was then removed, the cleaned carriers were dried at 105 °C for 24 h and re-weighed (M2). The biofilm mass per carrier was then calculated as the difference between M1 and M2.
VPSEM image acquisition and analysis
Variable pressure scanning electron microscope (VPSEM) imaging was used to measure in-situ biofilm thickness. VPSEM imaging does not require sample preparation and hence minimizes sample destruction prior to analysis [17]. The samples were analyzed a maximum of 4 h after being harvested and images were acquired at a pressure of 40 Pa using a Vega II-XMU SEM (Tescan USA Inc., Cranberry, PA). Five images of four replicate carriers (20 total images) were analyzed for thickness at ×60 magnification. The images were taken at random locations across the carrier surface. Biofilm thicknesses were quantified using Atlas image processing software (Tescan USA Inc., Cranberry, PA). VPSEM images in supplemental material (Fig. S-1).
DNA sequencing analysis
Approximately, 25 mg of wet biofilm was abraded from five carriers from each MBBR reactor into a 1.5 mL sterile Eppendorf tube. DNA was extracted from the biofilm using FastDNA® spin kit (MP Biomedicals, Santa Ana, CA) and the extracted DNA was stored at −80 °C until used for sequencing library construction.
Profiling the bacterial community was performed utilizing two-step polymerase chain reaction (PCR) strategy targeting the V6 hyper-variable region of the 16S rDNA gene as described previously [18]. The first step involved amplification of the V6 region and simultaneously adding 4–6 nucleotides barcodes and the Illumina sequencing adapters at 3′ ends of amplicons. At this step, unique combination of forward and reverse barcoded primers was used for each sample. The second PCR step involved adding the Illumina flow cell adapter at the 3′ ends of the first step PCR amplicons using PCRFWD1/PCRRVS1 primers pair. Template DNA and PCR component concentrations and amplification conditions for the first and second PCR were done as described elsewhere [18]. Following the second PCR amplification, amplicons were inspected on 2% agarose gel and purified with a Montage PCR96 cleanup kit (EMD Milipore, Billerica, MA). The purified amplicons were quantified using Quant-iT™ dsDNA HS Assay Kit (Life Technologies, Burlington, Canada) according to instructions, and equimolar amount of DNA amplicons from each sample were pooled together. The pooled sample was subjected to deep sequencing on a Illumina Hiseq2500 platform at The Center for Applied Genomics (TCAG, Toronto, Canada), which generates paired-end reads of 2 × 100 bases in length for downstream analysis.
The entire sequence analysis was disseminated using Bio Linux operating platform run on Dell Precision T7610 workstation. The 2 × 100 base paired-end reads were assembled using the Fast Length Adjustment of SHort reads (FLASH) software [19] then quality filtered using the fastq_quality_filter command from the Fastx toolkit with a minimum quality score of 20 over 90% of the sequences. Reads that passed the quality filter were demultiplexed and barcodes trimmed using Novobarcode [20]. The Quantitative Insights Into Microbial Ecology (QIIME) software, version 1.8 [21], was used to compute operational taxonomical unit (OTU) clustering with closed reference strategy at 97% sequence similarity. QIIME aligned the OTUs against the Greengenes database 13.8 utilizing UCLUST algorithm. Singleton OTUs were removed and the OTU table was normalized by dividing the biofilm mass in each sample group. The normalized OTU table was used to calculate the relative abundance of the bacterial taxa present in the biofilm and compute alpha and beta diversity. The differences in bacterial relative abundance were identified using Linear Discriminate Analysis (LDA) using Galaxy software [20, 22, 23]. Finally, fold changes were calculated by dividing the relative abundance of the significant taxa in the MBBR at day 28 by their corresponding relative abundance in the IFAS.
Statistical analysis
Statistical significance for the constituent and biofilm thickness data was tested using the t test with p values less than 0.05 considered significant. Statistical significance for the microbial community shifts were tested using a Kruskal–Wallis (KW) sum-ranked test followed by Dunn’s post-hoc and linear discriminant analysis (LDA) [24]. Bacterial taxa with LDA log-score of 2 and higher were selected. Correlation between ammonia concentration and AOB relative abundance was conducted by calculating Spearman’s rank correlation coefficient (R) for each time point. Finally, the trend in increasing AOB relative abundance was calculated with a linear regression curve (R 2).
Results and discussion
Ammonia removal kinetics
Following the harvesting of carriers from the carbon removal IFAS system with limited nitrification, the carriers were placed into the pilot post carbon removal nitrifying MBBR reactors, with the MBBR1 and MBBR2 operating at an initial wastewater temperature of 6 °C. After 22 h of operation in the pilot-scale post carbon removal MBBR system, ammonia removal rates of 103.4 ± 2.6 and 65.3 ± 3.1 gN/m3 d were achieved for MBBR1 and MBBR2, respectively, which corresponds to ammonia removal efficiencies of 72.5 and 74.5% (Fig. S-2). Operating in low temperatures traditionally impedes nitrification, as such these removal rates are comparable to conventional design start-up rates observed by previous studies [25].
The removal rate (RR) values of the MBBR reactors increased by an average value of 1.4 gN/m3 d per day until the 21st day of operation. Between the 18th and 21st day of operation, the system transitioned from DO mass transfer limited to ammonia mass transfer limited [25]. During the 8 days of ammonia mass transfer limited condition the average increases in RR of MBBR1 and MBBR2 were negligible where both reactors were at steady state and achieved removal efficiencies of 96% or greater (with effluent ammonia concentrations less than 1.0 mg-N/L in both reactors). Hence, even starting at a temperature of 6 °C, the system was capable of rapid start-up to achieve conventional removal rates offering significant advantages to the northern climate regions and mobile treatment units.
Ammonia, nitrite and nitrate were measured throughout the experimental phase to confirm nitrogen mass balances in both reactors (Fig. S-3). The maximum mass balance discrepancy between the influent and effluent nitrogen concentrations observed during the study was 15% (with less oxidized nitrogen being measured). An average mass balance error of 8.2 ± 6.6% was calculated over the experimental phase. Hence, the results demonstrate that nitrogen mass balances are relatively stable during start-up, acclimatization periods of operation in MBBR systems when seeded with carbon-removal IFAS carriers. As the operation of the post carbon removal MBBR reactors proceeded, the influent nitrogen was observed to be consistently higher than the oxidized nitrogen. The loss of nitrogen observed in this study is believed to be attributed to bacterial synthesis of nitrogen and laboratory error.
Solids and biofilm mass, thickness
Throughout the entire study the influent TSS concentration entering the pilot system remained stable at an average value of 7.3 ± 1.4 mg/L. Elevated effluent TSS values in both reactors were observed after the first 22 h of operation, which is indicative of an early sloughing event from the harvested carriers (Fig. 1). After 22 h of operation the TSS concentrations were 227.0 ± 27.3 and 164 ± 31.7 mg/L for MBBR1 and MBBR2, respectively. MBBR1 maintained a stable elevated TSS concentration of 32.7 ± 6.9 mg/L after the 22 h sloughing event until the 16th day of operation. On the 16th day of operation a second major sloughing event was recorded with a spike in TSS concentration to 85.3 ± 10.0 mg/L. Following the second sloughing event (day 16) the TSS concentration of MBBR1 became increasingly stable with an average of 16.8 ± 4.2 mg/L.
The effluent TSS concentrations of MBBR2 demonstrated more fluctuations in concentration than MBBR1 with three distinct sloughing events during the first 16 days of operation. The first observed sloughing event occurred after 22 h of operation (TSS of 164 ± 31.7 mg/L), the second sloughing event occurred on the 7th day of operation with a spike in TSS concentration to 204.7 ± 64.7 mg/L. The third sloughing event occurred on the 16th day of operation with a TSS concentration of 122.0 ± 40.7 mg/L. Following the third sloughing event (day 16) the TSS concentration of MBBR2 decreased daily to a final average of 21.0 ± 13.1 mg/L.
The additional sloughing event observed in MBBR2 as compared to MBBR1 along with the overall higher TSS concentrations in MBBR2 resulted in the average TSS concentration to be 1.6 times greater in MBBR2 as compared to MBBR1. The average TSS concentration for the 28 day start-up period was 51.0 mg/L for MBBR1 and 81.7 mg/L for MBBR2. This increase in produced effluent TSS by the sloughing of the seeded carriers that was observed in MBBR2 as compared to MBBR1 was expected due to the fact that the total mass of biofilm initially added into MBBR2 was higher 1.6 times than MBBR1. In particular, 1.6 times more carriers and hence more biofilm mass was added to MBBR2 than MBBR1 during the carrier filling process, which is precisely the average increase in effluent TSS values between the two reactors. It should be reiterated that once the biofilm and biomass became acclimatized in both reactors, the stabilized measured effluent TSS concentrations of both reactors were very similar with no statistical difference between the measured concentrations (p = 0.47). In total, approximately half of the initial biofilm was washed out in both reactors during the sloughing events prior to biofilm stabilization. As such, an initial solids separation phase or flow recirculation to the head of the plant will be required.
The biofilms transition throughout the start-up period with respect to thickness, mass and density was characterized for both MBBR reactors (Fig. 2). The biofilm thickness and mass measurements showed similar trends to the effluent TSS measurements for both reactors. In particular, MBBR1 and MBBR2 both showed decreases in biofilm thickness and mass during the first 19 days of operation while the biofilm was sloughing off the carriers and acclimatizing to post carbon removal treatment conditions. The dry density of biofilm in both reactors decreased during the first 12 days of operation; which was characterized by large sloughing events and a strong increase in ammonia removal efficiency. Although the dry density decreased during the initial start-up period, the dry density of the biofilms in both reactors remained within the range of densities expected for wastewater biofilms (30–60 kg/m3) [26]. The biofilm density of both reactors, following 12 days of operation, then showed a significant increase at 19 days of operation which corresponds to the period of stable effluent TSS concentrations, stable ammonia removal rates and the transition from a DO mass transfer limited biofilm to an ammonia mass transfer limited biofilm. Further, the dry density of the biofilm in both reactors, after 19 days of operation, returned to similar densities measured for the IFAS harvested carriers.
Both reactors showed a regrowth of the biofilm after 19 days of operation. The measured biofilm characteristics along with the effluent TSS measurements of this study indicate that the kinetics and the biofilm both stabilized after a period of approximately 19 days of operation. As such, the use of carriers from a carbon removal IFAS system to seed a post carbon removal MBBR treatment system with low carbon concentrations and high ammonia concentration did not exhibit failure due to severe shock effects on the biofilm and the system demonstrated a short acclimatization period.
Bacterial community shift
High-throughput Illumina sequencing generated a total of 29,752,892 sequences with an average of 367,320 ± 209,130 high quality reads per sample. Normalizing the high quality reads to biofilm mass yielded an average of 30,100 ± 21,320 high quality reads per sample per milligram of biofilm. Using the normalized reads, the Chao1 alpha diversity was not statistically significant between the IFAS biofilm (day 0) and each day operated as a post carbon removal MBBR system (Fig. S-4). This indicates that the IFAS (day 0) and MBBR share the same richness.
Principal coordinate analysis (PCoA) was performed based on the unweighted UniFrac distances from the biofilm microbiota at each time point (Fig. 3). Clustering occurred on the first two coordinate axes, PC1 and PC2, which account for 19.6 and 12.1% of the total variation, respectively. In addition, an analysis of similarity (ANOSIM) of each time period was applied where R = 1 indicating maximum dissimilarity. ANOSIM of the entire sample group showed the biofilm microbes clustered based on the time of operation as a post carbon removal MBBR (R = 0.43, p = 0.001). Concomitantly, comparing the IFAS biofilm (time 0) to the 28 d operation as a post carbon removal MBBR shows maximum dissimilarity (R = 1, p = 0.001) confirming the biofilm underwent a transition in the microbial structure with respect to the time of operation as a post carbon removal MBBR. The taxonomical shift at the phylum level and most abundant genus is presented in supplemental material (Fig. S-5, Table S-2).
Biofilm taxonomy
The IFAS biofilm was analyzed for microbial populations following harvesting from the IFAS full-scale treatment facility. The microbial community in the IFAS biofilm consisted of 36 phyla with 8 phyla representing 1% relative abundance or greater (Fig. S-5). The most prominent phylum in the sampled IFAS biofilm was Proteobacteria with 50.1 ± 23.2% relative abundance. Within the Proteobacteria phylum, there were 366 unique genera detected; with Novosphingobium (4.9 ± 2.7%), Zoogloea (4.5 ± 3.4%), Thiomonas (3.9 ± 2.9%) and Dechloromonas (1.9 ± 1.4%) being recorded as the most abundant. These bacteria are heterotrophic organisms that are often associated with aromatic compound degradation [27–29]. The second most abundant phylum in the sampled IFAS biofilm was Bacteroidetes with 38.9 ± 19.2% relative abundance. Within the Bacteroidetes phylum, there were 83 unique genera detected with Flavobacterium (7.5 ± 3.5%) and Fluviicola (2.2 ± 1.1%) as the most abundant genera. These genera of bacteria are also heterotrophic and are often observed in symbiotic roles with Proteobacteria and decompose high-molecular-mass organic matter [30]. The AOB population of the IFAS biofilm represented 2.0 ± 1.5% of the total identified bacterial cells embedded in the biofilm. The dominant genus of the AOB group was Nitrosomonas with Nitrosoccocus, Nitrosospira, and Nitrosovibrio also being detected at small percent abundances. NOBs were also detected in the IFAS biofilm. Their relative abundance was less than 7 × 10−3%. The only genus detected in the NOB group was Nitrospira.
During operation of the MBBR pilot plant, the post carbon removal MBBR biofilm samples were analyzed for microbial populations twice per week (9 total samples) during the 28 day start-up period. Throughout the entire start-up period, the same 36 phyla observed in the IFAS biofilm were detected in the MBBR biofilm. Along with the same observed phyla, the same 8 phyla that demonstrated a relative abundance of at least 1% in the IFAS biofilm were again observed at relative abundances equal to or greater than 1% in the MBBR biofilm (Fig. S-5). After operating for 4 days in the post carbon removal MBBR system, the bacterial community increased its average relative abundance of the Proteobacteria phylum (containing AOBs) from 50.1 ± 23.2 to 65.3 ± 2.2% (p = 0.50). The increase in Proteobacteria corresponded with a decrease in Bacteroidetes. The Bacteriodete phylum decreased from a relative abundance of 38.9 ± 19.2% to a relative abundance of 26.9 ± 1.2% (p = 0.98) (Fig. S-5). In the last 7 days of post carbon removal MBBR operation culminating with day 28, the microbial community did not significantly change with respect to the percent abundance of the Proteobacteria (p = 0.51) or Bacteroidete (p = 0.80) phyla; however, there was a significant increase in Acidobacteria. The Acidobacteria phylum represented 0.8 ± 0.2% relative abundance for each sampling period during the first 21 days of post carbon removal MBBR operation, which increased to 1.4 ± 0.2% (p = 0.02) relative abundance after 28 days of post carbon removal MBBR operation (Fig. S-5). Acidobacteria is a relatively newly classified phylum that shares similar genetic composition to Proteobacteria. The organisms of this phylum has proven difficult to culture and as such their overall function and specifically their function in wastewater biofilms is currently not well understood [31]. Species in the Acidobacteria phylum that have been cultured have been shown to be important to the biogeochemical cycle through their use of decomposing organic matter as a substrate [31, 32].
The most abundant heterotrophic genera observed in the IFAS biofilm decreased significantly after 21 days of post carbon removal MBBR operation (Table S-2). The dominant heterotrophic genera observed in the IFAS biofilm samples, Novosphingobium, Zoogloea, Thiomonas and Dechloromonas, Flavobacterium and Fluviicola, were still among the most prevalent genera in the post carbon removal MBBR biofilm after 4 days of operation (Table S-2). Conversely, the prominent heterotrophic organisms present in the IFAS system, were depleted after the period of 28 days. A sustained lack of carbon in the influent likely stressed the heterotrophic organisms to detach or lyse and be decomposed.
Differentially abundant taxa comparing the IFAS (day 0) biofilm and day 28 of post carbon removal MBBR operation identified Nitrosomonas, Myxococcales, Bryobacter and Nitrospira among the enriched bacteria in the MBBR system (Fig. 4). Nitrospira, a prominent NOB in wastewater and the dominant NOB in the biofilm of this study exhibited the highest enrichment with a fold change of 79.3. This large fold change is due to the near 0 relative abundance for the IFAS biofilm (day 0) and growth of Nitrospira in the MBBR biofilm. The post carbon removal nitrifying MBBR biofilm was shown to become enriched with Nitrospira without nitrite accumulation (Fig. 4, S-3). This lack of nitrite accumulation demonstrates the adaptability and robustness of biofilm subjected to rapid change.
Nitrosomonas, a prominent AOB in wastewater and the dominant AOB in the biofilm of this study, demonstrated an enrichment with a fold change of 2.46. Myxococcales demonstrated the second highest enrichment with a fold change of 2.95. Myxococcales is a rapid EPS forming bacteria which might have aided in stabilizing the nitrifying biofilm. Wastewater AOBs and particularly Nitrosomonas are poor EPS producing organisms. It is likely that this rapid EPS forming bacteria are partly responsible for the stabilization of the biofilm thickness and mass observed after the 19th day of operation (Fig. 2). Finally, Bryobacter, a decomposing Acidobacteria represented significant enrichment with a fold change of 2.86. As previously mentioned, after 28 days of post carbon removal MBBR operation, the biofilm had a significant increase in Acidobacteria (Fig. S-5). Bryobacter, a decomposing organism was the prominent genus in the Acidobacteria phylum. Bryobacter has been demonstrated to use polysaccharides, galacturonic and glucuronic acids as substrates that are formed during decomposition of organic matter [32]. It is possible that the Bryobacter consumed the lysed heterotrophic bacteria that remained in the biofilm after MBBR start-up. The natural propagation of such organisms has been proposed to aid in preventing a failure of the biofilm matrix under acclimatized conditions [14].
The AOB and NOB population percent abundances during the start-up of the MBBR pilot were compared to the kinetics of nitrification throughout the start-up phase of the system in this research (Fig. 5). The IFAS system was operating at a C:N ratio of 3:1. Ideal conditions for autotrophic growth range from C:N ratios of 0.5:1 to 1:1 [25], and as such the IFAS system demonstrated an oxidation of 27 ± 7% of the influent ammonia to nitrate. At the time of harvesting, the SRT of the suspended cells of the IFAS facility ranged between 4 and 6 days and temperature was 9.0 ± 0.1 °C. Hence, the moderate ammonia conversion rate observed at the facility was expected. It is most probable that the AOB and NOB populations of the biofilm, although low in percent abundances, were responsible for the limited nitrification and served as inoculum for the post carbon removal MBBR start-up.
The AOB population showed a linear increase (R 2 = 0.97) in relative abundance up to 8.1 ± 1.0% during the first 19 days of post carbon removal MBBR operation until the biofilm transitioned from DO or kinetic rate limited to ammonia mass transfer rate limited. In the DO or kinetic rate limited time period the decrease in ammonia concentration is correlated to the increasing AOB relative abundance (Spearman R = −0.90, p = 0.04). At this transitory point from a DO to an ammonia rate limited biofilm, the AOBs reached equilibrium within the biofilm at a percent abundance of 5.8 ± 0.7% of the total organisms. Nitrosomonas represented the majority of the AOB population with Nitrosoccocus, Nitrosospira, and Nitrosovibrio being detected at percent abundances less than 0.2%. The percent abundance of the NOB population was significantly lower than the AOB population, with an average relative abundance of 0.1 ± 0.1% after 19 days of operation. The relative abundance of the NOB population increased to 0.6 ± 0.3% after the full 28 day start-up period. There was not an observable correlation between ammonia concentration and NOB relative abundance (Spearman R = −0.62, p = 0.12). Nitrospira was the dominant NOB with Nitrobacter representing less than 1 × 10−4% of the microbial community.
The increase in AOB and NOB relative abundance through the 21 day acclimatization period was likely affected by the detachment of the biofilm during the acclimatization to high ammonia, low carbon conditions. In particular, the initial increase in relative abundance of AOBs and NOBs in the post carbon removal MBBR biofilms was likely due to the preferential detachment of heterotrophic organisms that dominated the outer layers of the IFAS biofilm (0 day). Based on previous studies, the heterotrophic communities are most prevalent in the outer layers of wastewater biofilm as they out-compete the slower growing autotrophic, nitrifying, organisms [33]. The detachment observed by elevated effluent TSS concentrations (Fig. 1) and a reduction in biofilm thickness and mass (Fig. 2) did not appear to destabilize the underlying nitrifying biomass as is evident by the maintained ammonia removal kinetics (Fig. S-2). After 19 days of operation, the biofilm successfully stabilized with respect to kinetic rates, biofilm morphology (thickness, mass, density) and the AOB relative abundance within the biofilm. The NOB relative abundance increased after the 19 days of operation and may have continued to increase in the biofilm due to their slow growth as compared with AOBs.
Conclusion
This study effectively demonstrates seeded carriers from a carbon removal IFAS system, which exhibited limited nitrification, rapidly acclimatized to MBBR post carbon removal nitrifying treatment at low temperatures. After 22 h of operation, the post carbon removal nitrifying MBBR removed 73.3 and 83.7% of the influent ammonia and reached steady state after 18 days of operation. This steady state operation corresponded to 96% of the influent ammonia (effluent concentrations less than 1 mg-N/L). The 18th day of operation also corresponded to stabilization of the biofilm and microbial community. It was observed the biofilm undergoes an initial sloughing period with rapid EPS producing bacteria, such as Myxococcales, stabilizing the biofilm matrix. The stabilized biofilm reached equilibrium relative abundance of AOBs and NOBs indicating the post carbon removal nitrifying MBBR has adapted to a true nitrifying system. As secondary IFAS treatment systems are the most common application of the IFAS technology, carriers from these systems may be more readily available for harvesting to seed MBBR nitrifying systems. Although seeding a full scale MBBR plant would present challenges with respect to the harvesting of a substantial volume of carriers, this study indicates the potential of using seeded carriers from an IFAS system to partially seed a new MBBR plant.
References
Canada Gazette (2012) Wastewater systems effluent regulations. Part II. 146(15)
Metcalf and Eddy (2003) Wastewater engineering: treatment and reuse, 4th edn. McGraw-Hill, New York
Maas CL, Parker WJ, Legge RL (2008) Detachment of solids and nitrifiers in integrated, fixed-film activated sludge systems. Water Environ Res 80(12):2202–2208
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623f–633
Delatolla R, Tufenkji N, Comeau Y, Gadbois A, Lamarre D, Berk D (2012) Effects of long exposure to low temperatures on nitrifying biofilm and biomass in wastewater treatment. Water Environ Res 84(4):328–338
Di Trapani D, Christensson M, Torregrossa M, Viviani G, Ødegaard H (2013) Performance of a hybrid activated sludge/biofilm process for wastewater treatment in a cold climate region: influence of operating conditions. Biochem Eng J 77:214–219
Hoang V, Delatolla R, Abujamel T, Mottawea W, Gadbois A, Laflamme E, Stintzi A (2014) Nitrifying moving bed biofilm reactor (MBBR) biofilm and biomass response to long term exposure to 1 °C. Water Res 49:215–224
Hibiya K, Tsuneda S, Hirata A (2000) Formation and characteristics of nitrifying biofilm on a membrane modified with positively-charged polymer chains. Coll Surf B Biointerfaces 18(2):105–112
Tsuneda S, Park S, Hayashi H, Jung J, Hirata A (2001) Enhancement of nitrifying biofilm formation using selected EPS produced by heterotrophic bacteria. Water Sci Technol 43(6):197–204
Wijffels RH, Englund G, Hunik JH, Leenen EJTM, Bakketun A, Gunther A, Obon de Castro JM, Tramper J (1994) Effects of diffusion limitation on immobilized nitrifying microorganisms at low temperatures. Biotechnol Bioeng 45:1–9
Sumino T, Nakamura H, Mori N, Kawaguchi Y (1992) Immobilization of nitrifying bacteria by polyethylene glycol prepolymer. J Ferment Bioeng 73(1):37–42
Sriwiriyarat T, Ungkurarate W, Fongsatitkul P, Chinwetkitvanich S (2008) Effects of dissolved oxygen on biological nitrogen removal in integrated fixed film activated sludge (IFAS) wastewater treatment process. J Environ Sci Health Part A Tox/Hazard Subst Environ Eng 43(5):518–527
Kwon S, Kim T, Yu GH, Jung J, Park H (2010) Bacterial community composition and diversity of a full-scale integrated fixed-film activated sludge system as investigated by pyrosequencing. J Microbiol Biotechnol 20(12):1717–1723
Telgmann U, Horn H, Morgenroth E (2004) Influence of growth history on sloughing and erosion from biofilms. Water Res 38(17):3671–3684
APHA A WEF (ed) (1995) Standard methods for the examination of water and wastewater. 19 edn. A.P.H.A.A.W.W.A.W.E. Federation, Washington DC, USA
Delatolla R, Berk D, Tufenkji N (2008) Rapid and reliable quantification of biofilm weight and nitrogen content of biofilm attached to polystyrene beads. Water Res 42:3082–3088
Delatolla R, Berk D, Tufenkji N (2009) In-situ characterization of nitrifying biofilm: minimizing biomass loss and preserving perspective. Water Res 43:1775–1787
Young B, Banihashemi B, Forrest D, Kennedy K, Stintzi A, Delatolla R (2016) Meso and micro-scale response of post carbon removal nitrifying MBBR biofilm across carrier type and loading. Water Res 91:235–243
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinform 27(21):2957–2963
Goecks J, Nekrutenko A, Taylor J, The Galaxy Team (2010) Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 11:R86
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Gonzalez Peña A, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatzunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Giardine B, Riemer C, Hardison RC, Burhans R, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J, Miller W, Kent J, Nekrutenko A (2005) Galaxy: a platform for interactive large-scale genome analysis. Genome Res 15:1451–1455
Blankenberg D, Von Kuster G, Coraor N., Ananda G, Lazarus R, Mangan M, Nekrutenko A, Taylor J (2010) Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol 19.10.1–19.10.21
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
WEF (2010) Design of municipal wastewater treatment plants: WEF manual of practice no. 8, 5th edn. McGraw-Hill, New York
Laspidou CS, Rittmann BE (2004) Evaluating trends in biofilm density using the UMCCA model. Water Res 38(14–15):3362–3372
Dugan P, Stoner D, Pickrum H (2006) The Genus Zoogloea. Prokaryotes 7:960–970
Bryan C, Marchal M, Battaglia-Brunet F, Kugler V, Lemaitre-Guillier C, Liévremont D, Bertin P, Arséne-Ploetze F (2009) Carbon and arsenic metabolism in Thiomonas strains : differences revealed diverse adaptation processes. BMC Microbiol 9(127): doi:10.1186/1471-2180-9-127
Kertesz M, Kawaski A (2010) In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66(4):1692–1697
Lee SH, Ka JO, Cho JC (2008) Members of the phylum Acidobacteria are dominant and metabolically active in rhizosphere soil. FEMS Microbiol Lett 285:263–269
Kulichevskaya IS, Suzina NE, Liesack W, Dedysh SN (2010) Bryobacter aggregatus gen. nov., sp. nov., a peat-inhabiting, aerobic chemo-organotroph from subdivision 3 of the Acidobacteria. Int J Syst Evol Microbiol 60(Pt 2):301–306
Lee LY, Ong SL, Ng WJ (2004) Biofilm morphology and nitrification activities: recovery of nitrifying biofilm particles covered with heterotrophic outgrowth. Bioresour Technol 95(2):209–214
Acknowledgements
The authors are grateful for the financial support from the Natural Science and Engineering Research Council of Canada and Veolia Water Technologies. The authors thank Daina Forrest, Walid Mottawea and James Butcher of the University of Ottawa for their technical support. The authors acknowledge the Saudi Arabian Cultural Bureau in Canada for scholarship contribution from King Abdulaziz University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Young, B., Delatolla, R., Abujamel, T. et al. Rapid start-up of nitrifying MBBRs at low temperatures: nitrification, biofilm response and microbiome analysis. Bioprocess Biosyst Eng 40, 731–739 (2017). https://doi.org/10.1007/s00449-017-1739-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1739-5