Abstract
Methanotrophs must become established and active in a landfill biocover for successful methane oxidation. A lab-scale biocover with a soil mixture was operated for removal of methane and nonmethane volatile organic compounds, such as dimethyl sulfide (DMS), benzene (B), and toluene (T). The methane elimination capacity was 211 ± 40 g m−2 d−1 at inlet loads of 330–516 g m−2 d−1. DMS, B, and T were completely removed at the bottom layer (40–50 cm) with inlet loads of 221.6 ± 92.2, 99.6 ± 19.5, and 23.4 ± 4.9 mg m−2 d−1, respectively. The bacterial community was examined based on DNA and RNA using ribosomal tag pyrosequencing. Interestingly, methanotrophs comprised 80 % of the active community (RNA) while 29 % of the counterpart (DNA). Types I and II methanotrophs equally contributed to methane oxidation, and Methylobacter, Methylocaldum, and Methylocystis were dominant in both communities. The DNA vs. RNA comparison suggests that DNA-based analysis alone can lead to a significant underestimation of active members.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfills are a significant source of the greenhouse gas methane, which accounted for approximately 5 % of the net world methane production in 2005 (Denman et al. 2007; Bogner et al. 2007). Biotic methane mitigation systems, e.g., landfill biocover, have been used to mitigate methane emissions from landfill sites where the emitted CH4 concentration is too low for flaring and energy recovery and/or installation of a gas collection system is not feasible. It is necessary for successful methane removal that methanotrophs become well established and active in landfill cover systems.
Landfill cover soil is often manipulated with inorganic and organic materials to improve the establishment and activity of methanotrophs (Park et al. 2008; Scheutz et al. 2009a). For instance, Park et al. (2008) reported that earthworm cast and powdered activated carbon were capable of enhancing the methanotrophic activity of landfill cover soils. Earthworm cast is a vermicomposting product, which released nutrients slowly into soil, resulting in a higher level of microbial activity and biomass (Chaoui et al. 2003). Earthworm cast can enhance methane oxidation, since its macroaggregates improve soil porosity (Bossuyt et al. 2005). Our recent reports showed that earthworm cast introduced into soil enhanced the methane oxidation potentials, since earthworm cast can be a significant source of methanotrophs (Moon et al. 2010). In addition, earthworm cast was shown to increase the population level of methanotrophs by more than 15-fold in a lab-scale biocover experiment (Kim et al. 2011). Furthermore, granular-activated carbon (GAC) holds great promise for use in enhancing methane oxidation of cover soil. Rivera-Utrilla et al. (2001) summarized that GAC enriches nutrient and oxygen concentrations, provides a protective habitat for microorganisms, and neutralizes toxic compounds. GAC also can improve aeration of the soil.
Methane oxidation capacity depends on the establishment of methanotrophs in biocover systems. The relative abundance of methanotrophs reflects their fitness and establishment, which should be considered when evaluating biocover materials. A number of molecular ecological techniques can be used to assess the relative abundance of methanotrophs within a microbial community. Pyrosequencing, a high-throughput sequencing technique, is perhaps the most powerful tool for microbial compositional studies to date (Gaidos et al. 2011; Will et al. 2010). The recently developed Genome Sequencer 454 FLX Titanium system produces greater than 1,000,000 sequence reads with >400-nt read lengths. Molecular community analyses have been largely dependent on DNA in environmental biotechnology areas. However, DNA-based analysis cannot separate actually active ones from a DNA pool. Ribosomal RNA-based community analysis has become popular in microbial ecology, since the rRNA content per cell can reflect growth activity (Wagner 1994; Vandecasteele et al. 2001). DNA-based analysis represents both active and dormant cells (naked DNA as well), while ribosomal RNA-based analysis is more likely to represent active cells (Gaidos et al. 2011; Felske et al. 1998). Comparison of ribosomal RNA- and DNA-based communities can determine if abundant taxa are active and if rare but active taxa are present (Gaidos et al. 2011).
In the present study, accurate information on the fitness of methanotrophs in a biocover was obtained by comparison of DNA- and RNA-based pyrosequencing libraries. In addition, the effect of biocover depth on bacterial community was evaluated. A lab-scale biocover was prepared using soil, earthworm cast, GAC, and saprolite (4:1:1:1, w/w). Landfill gas contains a wide variety of nonmethane organic compounds (NMOCs) at trace level (parts per million–parts per thousand), e.g., benzene (B), toluene (T) and dimethyl sulfide (DMS) (Kim et al. 2005; Bogner et al. 2003). The performance of the biocover for mitigating methane and malodorous and toxic NMOCs, such as DMS, B, and T, was evaluated. The bacterial community composition of the biocover was identified by ribosomal tag pyrosequencing, which was based on RNA and DNA.
Materials and method
Soil mixture preparation
Soil was collected from landfill cover soil at a depth of 10–30 cm (Gapyeong-gun, Kyungido, South Korea). Soil was sieved through a 2-mm mesh and stored at room temperature prior to use. GAC with a diameter of 4–8 mm was purchased from Jaeil Carbon, Co. Ltd., Yong-in, South Korea. A batch experiment confirmed that the GAC has no methane sorption capacity (data not shown). Earthworm cast (a moisture content of 8.86 %, a C:N ratio of 40, and an organic matter content of 56.29 %, pH 4.17, and a bulk density of 0.46) was obtained from the Nanji municipal sewage treatment plant, Goyang, Kyungido, South Korea (Moon et al. 2010). A consortium of mixed earthworms were cultivated with municipal sewage sludge for more than 2 years and air-dried for 6 months under natural conditions. Earthworm cast was sieved through a 2-mm mesh and stored at room temperature. Commercial saprolite with a diameter of 3 mm (Kyungseo Co. Ltd., Seoul, South Korea) was obtained and stored at room temperature. Saprolite was used to improve soil aeration. Soil, GAC, earthworm cast, and saprolite were completely mixed (4:2:1:1, w/w), and the moisture content was adjusted to 20 % with distilled water.
Lab-scale biocover
An acryl column was used to simulate landfill cover environments. A lab-scale biocover system was made up of two sections: the packing and ventilating sections with an inner diameter of 8 cm (Fig. 1). The packing bed of the packing section was 50 cm long (a volume of 2.5 L) and had a perforated plate at the bottom. It had gas sampling ports at 0, 10, 20, 30, 40, and 50 cm from the top. A gas inlet port was installed at the bottom. The ventilating section (a height of 15 cm) with air inlet and gas outlet ports was assembled on the packing column by rubber packing and six bolts/nuts. Gas tightness of the biocover system was verified with compressed air before use. The prepared soil mixture was filled up with the packing section (approximately 4 kg).
Commercial media bottles (500 mL) were used as volatilization chambers. DMS, B, and T were allowed to vaporize from their chambers as follows: 100 mL of DMS (99 %, Acros Organics Inc., Geel, Belgium), B (99.7 %, Kanto Chemical, Inc., Tokyo, Japan), or T (99.7 %, Kanto Chemical) solution was mixed with 100 mL of commercial corn oil to reduce excess vaporization. A synthetic gas of CH4/CO2 (2:3 v/v) was commercially purchased from Seoul Gas Inc., Seoul, South Korea. This synthetic gas was passed through a 50-cm long humidifier and then combined with DMS, B, and T that had vaporized from the volatilization chambers, before entering the biocover. DMS, B, and T were added periodically to maintain the desired concentrations. This landfill gas (CH4, CO2, DMS, B, and T) was continuously introduced into the biocover. Compressed air was entered into the top of the ventilating section at a flow rate of 100 mL min−1. Gas flows were controlled using commercial flow meters (air flow meter, Dwyer Inc., Michigan City, USA and CH4 flow meter, Kofloc Inc., Kyoto, Japan).
During a 40-day acclimation period, methane, DMS, B, and T were fed at concentrations of 400,000, 10–700, 10–420, and 10–80 ppm, respectively, at a flow rate of 5 mL min−1 (a space velocity of 0.12 h−1). After the acclimation period, methane, DMS, B, and T were fed at concentrations of 400,000, 115–3,000, 100–800, and 20–150 ppm, respectively, at a flow rate of 5 mL min−1. Air was entered into the headspace of the ventilating section at a flow rate of 100 mL min−1. The biocover system was operated at 20 ± 5 °C. Soil moisture was maintained by adding 200 mL of distilled water every 7 days. Methane oxidation efficiencies were calculated from methane fluxes of the inlet and outlet of the biocover system. Methane flux at the inlet was calculated from the inlet flow rate and methane concentration. Methane flux out of the system was calculated from the outlet flow rate and methane concentration. DMS and B/T oxidation efficiencies also were calculated by the same calculation method.
Gas analysis
Methane, DMS, B, and T concentrations were monitored using gas chromatography (GC, 6850N, Agilent Technologies Inc., Santa Clara, USA), which was equipped with a flame ionization detector (FID) and a wax column (30 m × 0.32 mm × 0.25 μm, Supelco Inc., Bellefonte, USA), as previously described by Lee et al. (2010). O2 and N2 concentrations were monitored using GC (7890A, Agilent), equipped with a capillary column (30 m × 0.53 mm × 28 μm, HP Molesieve 5A, Agilent), and a thermal conductivity detector (TCD). The oven, injector, and detector temperatures were set at 50, 200, and 250 °C, respectively. CO2 concentrations were monitored using GC (6890N, Agilent) equipped with a HP-PLOT/Q column (30 m × 0.53 mm × 40 μm) and a TCD. The oven, injector, and detector temperatures were set at 60, 100, and 250 °C, respectively.
Standard curves for gas analyses were established using CH4, DMS, B, T, N2, O2, and CO2 standard gases. Pure CH4, N2, O2, and CO2 gases were commercially purchased and used as standard gases. The concentrations of the CH4 standard ranged from 250 to 75,000 or 50,000 to 450,000 ppm. N2, O2, and CO2 concentration standards ranged from 2,500 to 300,000, 9,000 to 1,000,000, and 200 to 666,667 ppm, respectively. DMS, B, T, and MT standard gases were prepared as follows: serum bottles (600 mL) were washed several times and oven-dried. The bottles were sealed with a butyl-rubber stopper and an aluminum cap. Two microliters of DMS, B, or T solution were added with a microsyringe into each bottle. The bottles were shaken until the DMS, B, and T solutions were completely volatilized. The detection limits were 20, 25, 10, 50, 0.5, 1, and 1 ppm for O2, N2, CH4, CO2, DMS, B, and T, respectively.
Nucleic acid extraction
Soil mixtures were collected from depths of 0–10, 20–30, and 40–50 cm from the top at the end of the experimental period. They were immediately frozen at −70 °C. Genomic DNA was extracted from 0.5 g soil mixture in duplicate. DNA was extracted using a NucleoSpin Soil kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) with a modification wherein the samples were disrupted using a BeadBeater-8 system (BioSpec Inc., Bartlesville, USA) at 5,000 rpm for 30 s. DNA was eluted in 100 μL of the elution buffer and stored at −20 °C prior to use. DNA was quantified using an ASP-2680 spectrophotometer (ACTGene Inc., Piscataway, USA).
RNA was extracted from the top layer (0–10 cm) in duplicate. For RNA extraction, 0.5 g of the soil mixture were added to 2-mL microcentrifuge tubes containing 0.1 mm glass beads and 1 mm zirconia/silica beads (0.5 g each). Equal amount (0.5 mL) of hexadecyltrimethylammonium bromide (CTAB) buffer (10 % CTAB in 0.7 M NaCl and 240 mM potassium phosphate buffer (pH8)) and phenol:chloroform:isoamylalcohol (25:24:1) was then added. Samples were disrupted using a BeadBeater-8 system (BioSpec) at 5,000 rpm for 30 s and centrifuged at 12,000 × g for 5 min at 4 °C. The top aqueous layers were transferred to new 1.5-mL tubes, and an equal volume of chloroform:isoamyl alcohol (24:1) was added to the tubes. After mixing well, the tubes were centrifuged at 12,000 × g for 15 min at 4 °C. The top aqueous layers were transferred to new 1.5-ml tubes, and 0.1 volumes of 3 M NaOAc (pH 5.2) and 2.5 volumes of 100 % EtOH were added to the tubes. The samples were incubated at −20 °C for at least 2 h and centrifuged at 16,000 × g for 15 min at 4 °C. Pellets were washed with 70 % EtOH, and the tubes were centrifuged at 16,000 × g for 5 min at 4 °C. Pellets were air-dried briefly and resuspended in 50 μL 0.1 % DEPC-treated water. RNA was immediately purified using a Qiagen RNeasy Mini kit (Qiagen Inc., Valencia, USA) with a RNase-Free DNase set (Qiagen) according to the manufacturer’s instructions. RNA was quantified using an ASP-2680 spectrophotometer (ACTGene). All RNAs were stored at −70 ° C before use. DNA contamination was confirmed by PCR with a 340F-805R primer set which information is shown below. The 50-μL mixtures contained 5 μL of 10× PCR buffer (GenenMed Inc., Seoul, South Korea), 1 μL of each primer (10 μM), 1 U of Ace Taq polymerase (GenenMed), 4 μL of 2.5 mM dNTPs, and 1 μL of RNA. The reaction was performed in a 2700 GeneAmp® PCR system (Applied Biosystems Inc., Foster, USA). An initial denaturation step at 95 °C for 3 min and 25 cycles of denaturation at 94 °C for 30 s, primer annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s were performed, which was followed by the final elongation step at 72 °C for 5 min. PCR results were confirmed by 2 % agarose gel electrophoresis. No positive results were obtained from RNA (data not shown).
Reverse-transcription PCR
RNA was reverse-transcribed using a Qiagen OMNI RT kit (Qiagen) according to the manufacturer’s instructions. Each reaction mixture (20 μL) consisted of 1× RT Sensiscript buffer, each deoxynucleoside triphosphate at a concentration of 0.5 mM, random hexamer at 10 μM, 10 U of RNase inhibitor (RNaseOUT™, Invitrogen Inc., Grand Island, USA), 1 μL of RT-Sensiscript reverse transcriptase, and 10 μL of RNA.
Polymerase chain reaction (PCR) for pyrosequencing
Pyrosequencing analysis was performed in duplicate on DNA and cDNA from the top layer (0–10 cm). This analysis also was performed on DNA from the middle (20–30 cm) and bottom (40–50 cm) soils (no duplication). Thus, there were six pyrosequencing libraries.
For PCR, the primer set 340F (5′-TCCTACGGGAGGCAGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) was used to amplify the 340–805 region of the 16 S rRNA gene (positions based on Escherichia coli) containing the V3 and V4 regions. The 340F and 805R primers cover approximately 90 % of the sequences with a 300–850 region (based on the 16S rRNA gene of E. coli) in the RDP database (http://rdp.cme.msu.edu). Six different composite primer sets were made based on the 340F-805R set for multiplex pyrosequencing; the composite forward primer 5′-CCATCTCATCCCTGCGTGTCTCCGAC-TCAG-NNNNNNNNNN-TCCTACGGGAGGCAGCAG-3′ and the composite reverse primer 5′-CCTATCCCCTGTGTGCCTTGGCAGTC-TCAG-GACTACHVGGGTATCTAATCC-3′, where the underlined sequence is the Genome Sequencer FLX Titanium primer A, the sequence in italics is the four-base library key, NNNNNNNNNN is the 10-bp multiplex identifier tag, and the last is the primer targeting the 16S rRNA gene. For each sample, four independent PCR mixtures were prepared in parallel to avoid PCR bias. The 50-μL mixtures contained 5 μL of 10× PCR buffer (GenenMed), 10 μg of bovine serum albumin, 2 μL of each composite primer (10 μM), 1 U of Ace Taq polymerase (GenenMed), 4 μL of 2.5 mM dNTPs, and 2.5–3 μL of template DNA. The reactions were performed using a 2700 GeneAmp® PCR system (Applied Biosystems). An initial denaturation step at 95 °C for 3 min and 25 cycles of denaturation at 94 °C for 45 s, primer annealing at 50 °C for 45 s and elongation at 72 °C for 45 s were performed, which was followed by a final elongation step at 72 °C for 5 min. Four PCR products were combined, and the PCR products were subjected to electrophoresis using 2 % low melting agarose gel. The expected size of the PCR products was about 530 bp. Agarose gels containing the PCR products were excised using a scalpel and then purified using the QIAquick Gel extraction kit (Qiagen), as specified by the manufacturer. Concentrations of the purified DNAs were measured using an ASP-2680 spectrophotometer (ACTGene). Equal amounts of the purified DNAs were combined in a single tube and sent to Macrogen Incorporation (Seoul, South Korea) to be run on a Genome Sequencer 454 FLX Titanium system (Roche Diagnostics Inc., Mannheim, Germany).
Pyrosequencing data analysis
For high-quality sequences, primer sites of sequences were trimmed, and low-quality and chimera sequences were excluded. Primer sites and low-quality sequences (length <400 nt, average quality score <25, and with an ambiguity) were removed using the RDP pyrosequencing pipeline (Cole et al. 2009). Any possible chimeras were removed using Black Box Chimera Check software (Gontcharova et al. 2010) at the default setting. The RDP pyrosequencing pipeline was used for analyzing pyrosequencing data in this study unless otherwise stated. Operational taxonomic units (OTU) were determined at 3 % dissimilarity, and Chao1 richness estimates (Chao1) and Shannon–Weaver diversity indices (H′) were calculated. Pyrosequencing reads were taxonomically assigned using the RDP classifier of the pipeline with bootstrap values more than 80 %. The RDP classifier provides rapid and independent taxonomic classifications from domain to genus. The classification results were analyzed using MEGAN software version 4.50 (Huson et al. 2007). Bacterial communities were compared using the function of “Goodall-normalized” in the MEGAN software.
Reads assigned to methanotrophic genera (Semrau et al. 2010; Iguchi et al. 2011) belonging to type I (ɤ-Proteobacteria) and type II methanotrophs (α-Proteobacteria) from the pyrosequencing libraries. OTUs were determined at 1 % dissimilarity, and Chao1 and H′ were calculated. For further classification of methanotrophic genera to species, sequences representing OTUs were compared with those of bacterial-type strains using the EzTaxon server version 2.1 (http://www.eztaxon.org) (Chun et al. 2007). Sequences were identified at species level when the similarity was more than 99 %. If not, sequences were identified at the genus level. The pyrosequencing reads obtained in this study were deposited into the DNA Data Bank of Japan (DDBJ) Sequence Read Archive (http://trace.ddbj.nig.ac.jp/dra) under accession no. DRA000520.
Results
Lab-scale biocover performance
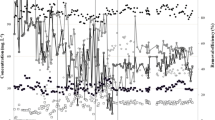
During a 40-day acclimation period, the methane removal efficiency increased from 0 to 30 %, while DMS, B, and T were completely removed after 30 days (data not shown). Figure 2 shows the inlet and outlet concentrations and removal efficiencies of methane, DMS, B, and T after the acclimation period. The methane removal efficiency was 47.3 ± 8.4 %. The methane elimination capacity (EC) was 211.0 ± 40.4 g m−2 d−1 at inlet loads of 330.1–515.8 g m−2 d−1, with a maximum level of 273.1 g m−2 d−1 at an inlet load of 501.0 g m−2 d−1. DMS, B, and T were completely removed at inlet loads of 5.3 ± 2.2, 2.4 ± 0.5, and 0.6 ± 0.1 g m−2 d−1, respectively.
Gas profiles
Profiles of CH4, N2, CO2, O2, DMS, B, and T were found to be stable during the 27-day experimental period. Figure 3 shows depth profiles on days 2 and 26. The N2 profile indicated that ambient air diffused into the whole column. Most of the O2 was consumed at the top layer (0–10 cm), but trace O2 (<7,500 ppm) was present in the bottom layer (10–50 cm). Methane and CO2 concentrations declined from the bottom to the top. The CO2/CH4 (mole to mole) ratios were 8.36, 1.92, 1.83, 1.71, 1.49, and 1.40 on day 2 and 8.05, 3.07, 2.21, 2.06, 1.74, and 1.68 on day 26 at 0, 10, 20, 30, 40, and 50 cm from the top, respectively. DMS, B, and T were completely removed at the bottom layer (40–50 cm).
Comparison of DNA and RNA bacterial communities in the top layer
DNA and RNA were extracted from the top layer (0–10 cm) in duplicate, and ribosomal tag pyrosequencing was performed. High-quality sequences (>400 nt, average quality score >25, and no ambiguity and possible chimeras) were obtained after filtering steps. DNAtop 1 and 2 and RNAtop 1 and 2 libraries contained 1,240 sequences (average read length, 416) and 782 (421), and 3,152 (412) and 842 (415), respectively (Table 1). The numbers of observed OTU, Chao1, and H′ indices are listed at a sequence divergence of 3 % in Table 1. Chao1 and H′ of the DNAtop community did not significantly differ from those of the RNAtop community (p > 0.05). Mean values of Chao1 and H′ were 133.3 and 2.44 for the DNAtop community and 75.8 and 1.93 for the RNAtop community. A phylogenetic comparison demonstrated replicate homogeneity as well as difference between the DNAtop and RNAtop communities (Fig. 4a). The genus Pseudoxanthomonas dominated the DNAtop community, followed by Methylocystis, Methylocaldum, Hyphomicrobium, Methylobacter, Rhodanobacter, and Dokdonella (Fig. 4b). In contrast, methanotrophs, such as Methylocystis, Methylocaldum and Methylobacter, dominated the RNAtop community, followed by nonmethanotrophs.
Comparison of DNA and RNA methanotrophic communities in the top layer
A total of six methanotrophic genera (Methylobacter, Methylocaldum, Methylomonas, Methylosarcina, Methylocystis, and Methylosinus) were observed from the biocover. These pyrosequencing reads were retrieved from the DNAtop and RNAtop libraries, and their methanotrophic communities were analyzed. Sequence numbers, OTU, Chao1, and H′ are listed at a sequence divergence of 1 % in Table 1. Chao1 and H′ values of the DNAtop methanotrophic community did not differ from those of the active methanotrophic community (p > 0.05). Mean values of Chao1 and H′ were 52.9 and 1.91 for the DNAtop methanotrophic community and 163.7 and 2.26 for the counterpart. Methanotrophs comprised approximately 80 % of the active bacterial community (RNAtop), while only approximately 29 % of the bacterial community (DNAtop) (Fig. 5a). Methanotrophs were more active than nonmethanotrophs in the top layer (p < 0.05). The abundance of type I methanotrophs did not differ from that of type II in both communities (p > 0.05). Methylobacter, Methylocaldum, and Methylocystis were dominant in both methanotrophic communities, and their relative abundances were comparable between the methanotrophic communities (Fig. 5b).
Comparison of bacterial communities (DNA) in the top, middle, and bottom layers
DNA-based bacterial communities in the middle (20–30 cm) and bottom (40–50 cm) layers were also analyzed. DNAmiddle and DNAbottom libraries contained 1,870 (average read length, 422) and 904 (422) sequences, respectively. The numbers of OTUs, Chao1, and H′ of the DNAmiddle library were 77, 94.25, and 2.14, respectively, at a sequence divergence of 3 %. Those of the DNAbottom library were 84, 119.00, and 2.99. Proteobacteria comprised 93.5 and 81.1 % of the DNAmiddle and DNAbottom communities, respectively, followed by Firmicutes. The DNAmiddle community included three dominant orders: Xanthomonadales (59.5 % of the whole community), Rhizobiales(16.1 %), and Methylococcales (13.3 %). The DNAbottom library included Xanthomonadales (38.7 %), Methylococcales (30.4 %), and Rhizobiales (7.8 %). The genus Pseudoxanthomonas dominated the DNAmiddle community, followed by Methylocystis, Methylobacter, Rhodanobacter, Hyphomicrobium, and Methylocaldum (Fig. 6). The DNAbottom community included Rhodanobacter, Methylobacter, Methylocaldum, Pseudoxanthomonas, and Hyphomicrobium in order of abundance.
Four methanotrophic genera (Methylobacter, Methylocaldum, Methylosarcina, and Methylocystis) were observed from the middle and bottom layers. The abundance of methanotrophs was greater in the top layer (approximately 29 %) than the middle and bottom layers (18.0 and 18.5 %, respectively). Types I and II methanotrophs were similarly abundant in the DNAmiddle methanotrophic community (42.6 % vs. 57.4 %, respectively) with Methylocystis (type II, 57.4 % of the methanotrophic community), Methylobacter (I, 28.6 %), and Methylocaldum (I, 14.0 %). Unlike the upper layers, type I members were predominant in the bottom layer (92.2 %), with Methylocaldum (I, 53.9 %), Methylobacter (I, 37.1 %), Methylocystis (II, 7.1 %), and Methylosarcina (I, 1.2 %).
Discussion
A lab-scale biocover was operated with a soil mixture, and its bacterial community was analyzed using pyrosequencing. The soil mixture holds promise for use as a landfill biocover material for mitigation of methane and NMOCs, since it favored habitation of the relevant microorganisms. A comparison between the results of the DNA- and RNA-based communities demonstrated that DNA results alone can lead to underestimation of active members. For instance, methane-oxidizing bacteria were estimated to be 29 % and 80 % in the DNA and RNA communities, respectively. Therefore, the RNA approach along with DNA is recommended for analysis of functionally active methanotrophs.
Soil was amended with earthworm cast, GAC, and saprolite to provide an optimum condition for habitation of methane-oxidizing bacteria. A lab-scale biocover with the soil mixture was operated for 27 days at a space velocity of 0.12 h−1 to remove methane, DMS, B, and T. The methane EC was 211.0 ± 40.4 g m−2 d−1, with a maximum level of 273.1 g m−2 d−1. Park et al. (2008) reported a maximum EC of 266.6 g-CH4 m−2 d−1 from a biocover with soil and earthworm cast (3:2, w/w) at a space velocity of 0.027 h−1. Scheutz et al. (2009b) reported a EC of 161 g-CH4 m−2 d−1 from a biocover consisting of compost and wood chips (1:1), 110 g-CH4 m−2 d−1 for a commercial compost product and 29 g-CH4 m−2 d−1 for sand and compost (5:1) at a space velocity of 0.029–0.032 h−1. Einola et al. (2008) found that the EC was less than 82.3 g-CH4 m−2 d−1 from biocovers containing mechanically–biologically treated municipal solid wastes at a space velocity of 0.013–0.034 h−1. Compared with the previous studies, the space velocity was at least three to fourfold faster and the removal capacity was greater or comparable, indicating a greater removal capacity per unit volume. Thus, our system is more efficient with regards to methane removal. Vertical profiles of CH4, N2, CO2, O2, DMS, B, and T were found to be stable during the period. The rapid O2 consumption and greater CO2/CH4 ratio indicated that methane oxidation activity was greatest at the top layer (0–10 cm) (Gebert et al. 2011). DMS, B, and T were completely removed at the bottom layer (40–50 cm) during the experimental period. Thus, the presence of the NMOCs presumably did not affect methane oxidation performance. Consistently, earlier studies showed that the biodegradation of volatile organic compounds actively occurred below the top layer in active methanotrophic biocovers (Scheutz and Kjeldsen 2005, 2003). The observed removal efficiencies and ECs of B/T are greater than a previous observation by Scheutz and Kjeldsen (2005) who reported ECs of 0.18 and 0.12 g m−2 d−1 with removal efficiencies of 26 and 44 % for B and T, respectively. These results indicated that the soil mixture is promising for concurrent mitigation of methane and NMOCs as a landfill cover material.
Microbial community with the highest CH4 oxidation activity was examined based on DNA and RNA, using ribosomal tag pyrosequencing. DNA- vs. RNA-based pyrosequencing libraries likely represent communities of numerically abundant members, including the active and dormant vs. active members, respectively. The DNAtop community was found to be richer and more diverse than the RNA-based counterpart, which is consistent with a previous observation (Gaidos et al. 2011). Differences between the DNA and RNA communities have been commonly observed (Weinbauer et al. 2002; Gaidos et al. 2011). This soil mixture exhibited lower levels of richness and diversity than other soil environments (Gaidos et al. 2011; Will et al. 2010) due to the favorable condition for methanotroph growth. There was no difference between the DNA and RNA communities at the phylum level, and Proteobacteria was shown to comprise more than 90 % of the communities, followed by Actinobacteria. A difference was observed on the order level. The phylum Proteobacteria included three dominant orders: Rhizobiales (α-Proteobacteria), Xanthomonadales (γ), and Methylococcales (γ). The DNAtop community included Xanthomonadales, Rhizobiales, and Methylococcales in order of abundance, while Rhizobiales and Methylococcales were more abundant than Xanthomonadales in the RNAtop community. The observed methanotrophs in this study belong to Rhizobiales and Methylococcales. Methanotrophs were more abundant than nonmethanotrophs in the active community, but less in the DNA counterpart. Therefore, Xanthomonadales was most and least abundant in the DNAtop and RNAtop communities, respectively.
Interestingly, methanotrophs were predominant, making up approximately 80 % of the active bacterial community (RNAtop). In contrast, methanotrophs comprised only approximately 29 % of the bacterial community (DNAtop). To the best of our knowledge, this is the first report to determine the predominance of methanotrophs in biocover systems. Our findings demonstrated that methanotrophs can exhibit a significant competitive ability in complex microbial communities of biocover systems. The presence of different physiological statuses (i.e., dormant vs. active cells and different growth phases) can cause differences in the abundances of the DNA- and RNA-based community members (Fey et al. 2004; Vandecasteele et al. 2001). In addition, the multiplicity of 16S rRNA operons (1–15) within bacterial strains (Rainey et al. 1996; Klappenbach et al. 2000) could lead to a bias in the bacterial abundance at the DNA level. Recent genome studies have revealed that methanotrophs contain a single 16S rRNA operon, such as Methylocystis sp. ATCC49242 (Accession number: AEVM00000000), Methylosinus trichosporium OB3b (ADVE00000000), and Methylobacter tundripaludum SV96 (AEGW00000000), except for Methylomonas methanica MC09 (NC015572), which contains three operons, indicating that methanotrophs might be underestimated at the DNA level.
Phylogeny and physiology simply classify methanotrophs into types I and II groups, which belong to γ- and α-Proteobacteria, respectively (Semrau et al. 2010). Types I and II methanotrophs were similarly abundant in both communities and thus, equally contributed to the methane oxidation in the biocover, which were consistent with previous observations that types I and II were equally present in a landfill cover soil (Héry et al. 2008) and similarly contributed to the methane oxidation in field soil (Henckel et al. 2000). Methylobacter, Methylocaldum, and Methylocystis dominated both methanotrophic communities. Relative abundances of the observed methanotrophic genera were comparable between the methanotrophic communities, indicating that the observed methanotrophs at the DNA level were active in the biocover. Pseudoxanthomonas and Hyphomicrobium were dominant nonmethanotrophic genera at the top layer, known to be heterotrophic and restrictedly methylotrophic, respectively. Both genera are commonly associated with methanotrophs in methane oxidation systems (Nikiema et al. 2005; Kim et al. 2012), but the relationship between them has not been elucidated. During methane oxidation, methanotrophs incorporate methane as cellular carbon compounds and produce metabolites such as methanol, formaldehyde, and formate (Hanson and Hanson 1996). These carbon compounds are utilized by heterotrophic bacteria including methylotrophs, other than methanotrophs (Qiu et al. 2009). It was speculated that both genera were cross-fed by methanotrophs, resulting in the high population density in the biocover.
Microbial communities of the middle (20–30 cm) and bottom (40–50 cm) layers were analyzed based on DNA to investigate effects of the NMVCs on microbial communities. The DNA pyrosequencing results from the top, middle, and bottom soils indicated that Proteobacteria dominated the biocover system, with three dominant orders: Xanthomonadales, Rhizobiales, and Methylococcales. Dominant genera included Pseudoxanthomonas, Methylocystis, Methylobacter, Rhodanobacter, Hyphomicrobium, and Methylocaldum. However, community comparison revealed that the DNAbottom community was distinct from those of the upper layers (data not shown). For instance, Pseudoxanthomonas made up less than 10 % of the bottom soil and more than 40 % of the upper soils, while the Rhodanobacter population increased toward the bottom. The methanotrophic community of the bottom also was distinct from those of the upper layers (data not shown). For example, type I members were predominant in the bottom layer in contrast to the even distribution of types I and II methanotrophs in the upper layers. The fact that CH4 and CO2 concentrations were similar between the middle and bottom layers indicated that the high concentrations of CH4 and CO2 were not likely to be responsible for the community difference and predominance of type I members at the bottom layer. Therefore, the presence of NMOCs might change the bacterial and methanotrophic communities. Similarly, Lee et al. (2010) reported that the presence of B/T could reduce the type II population density, and Saari and Martikainen (2003) showed that DMS and DMSO inhibit the methanotrophic activity. In addition, methanotrophic community has been shown to be modified by volatile organic compounds (Gebert et al. 2008).
There were a number of potential bacterial genera for degrading NMOCs. For example, some Rhodanobacter and Hyphomicrobium members are hydrocarbon degraders (Kanaly et al. 2002; Nadalig et al. 2011). In addition, methanotrophs are able to degrade hydrocarbons (Semrau et al. 2010) and transform DMS to DMSO (Fuse et al. 1998). A sulfur mass balance showed that DMS was converted to sulfate (80.2 % of the loaded DMS), DMSO (39.9 %), MT (0 %), and H2S (0 %) in the biocover (data not shown). Therefore, both methanotrophs and nonmethanotrophs might be responsible for the transformation of DMS.
References
Bogner J, Chanton J, Blake D, Morcet M, Kjeldsen P (2003) Comparative oxidation and net emissions of methane and selected non-methane organic compounds in landfill cover soils. Environ Sci Technol 37:5150–5158
Bogner J, Ahmed MA, Diaz C, Faaij A, Gao Q, Hashimoto S, Mareckova K, Pipatti R, Zhang T (2007) Waste management. In: Metz B, Davidson OR, Bosch PR, Dave R, Mey LA (eds) Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change, 2007. Cambridge University Press, Cambridge
Bossuyt H, Six J, Hendrix PF (2005) Protection of soil carbon by microaggregates within earthworm casts. Soil Biol Biochem 37:251–258
Chaoui HI, Zibilske LM, Ohno T (2003) Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biol Biochem 35:295–302
Chun J, Lee J-H, Jung Y, Kim M, Kim S, Kim BK, Lim Y-W (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X et al (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, 2007. Cambridge University Press, Cambridge
Einola J-KM, Karhu AE, Rintala JA (2008) Mechanically–biologically treated municipal solid waste as a support medium for microbial methane oxidation to mitigate landfill greenhouse emissions. Waste Manage 28:97–111
Felske A, Wolterink A, Van Lis R, Akkermans ADL (1998) Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands). Appl Environ Microbiol 64:871–879
Fey A, Eichler S, Flavier S, Christen R, Hofle MG, Guzman CA (2004) Establishment of a real-time PCR-based approach for accurate quantification of bacterial RNA targets in water, using Salmonella as a model organism. Appl Environ Microbiol 70:3618–3623
Fuse H, Ohta M, Takimura O, Murakami K, Inoue H, Yamaoka Y, Oclarit JM, Omori T (1998) Oxidation of trichloroethylene and dimethyl sulfide by a marine Methylomicrobium strain containing soluble methane monooxygenase. Biosci Biotechnol Biochem 62:1925–1931
Gaidos E, Rusch A, Ilardo M (2011) Ribosomal tag pyrosequencing of DNA and RNA from benthic coral reef microbiota: community spatial structure, rare members and nitrogen-cycling guilds. Environ Microbiol 13:1138–1152
Gebert J, Stralis-Pavese N, Alawi M, Bodrossy L (2008) Analysis of methanotrophic communities in landfill biofilters using diagnostic microarray. Environ Microbiol 10:1175–1188
Gebert J, Rower IU, Scharff H, Roncato CDL, Cabral AR (2011) Can soil gas profiles be used to assess microbial CH4 oxidation in landfill covers? Waste Manage 31:987–994
Gontcharova V, Youn E, Wolcott RD, Hollister EB, Gentry TJ, Dowd SE (2010) Black box chimera check (B2C2): a windows-based software for batch depletion of chimeras from bacterial 16S rRNA gene datasets. Open Microbiol J 4:47–52
Hanson R, Hanson T (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Henckel T, Roslev P, Conrad R (2000) Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ Microbiol 2:666–679
Héry M, Singer AC, Kumaresan D, Bodrossy L, Stralis-Pavese N, Prosser JI, Thompson IP, Murrell JC (2008) Effect of earthworms on the community structure of active methanotrophic bacteria in a landfill cover soil. ISME J 2:92–104
Huson DH, Auch AF, Qi J, Schuster SC (2007) MEGAN analysis of metagenomic data. Genome Res 17:377–386
Iguchi H, Yurimoto H, Sakai Y (2011) Methylovulum miyakonense gen. nov., sp. nov., a type I methanotroph isolated from forest soil. Int J Syst Evol Microbiol 61:810–815
Kanaly RA, Harayama S, Watanabe K (2002) Rhodanobacter sp. strain BPC1 in a benzopyrene-mineralizing bacterial consortium. Appl Environ Microbiol 68:5826–5833
Kim K-H, Choi YJ, Jeon EC, Sunwoo Y (2005) Characterization of malodorous sulfur compounds in landfill gas. Atmos Environ 39:1103–1112
Kim TG, Moon K-E, Lee E-H, Choi S-A, Cho K-S (2011) Assessing effects of earthworm cast on methanotrophic community in a soil biocover by concurrent use of microarray and quantitative real-time PCR. Appl Soil Ecol 50:52–55
Kim TG, Lee E-H, Cho K-S (2012) Microbial community analysis of a methane-oxidizing biofilm using ribosomal tag pyrosequencing. J Microbiol Biotechnol 22:360–370
Klappenbach JA, Dunbar JM, Schmidt TM (2000) rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol 66:1328–1333
Lee E-H, Park H, Cho K-S (2010) Characterization of methane, benzene and toluene-oxidizing consortia enriched from landfill and riparian wetland soils. J Hazard Mater 184:313–320
Moon K-E, Lee S-Y, Lee SH, Ryu HW, Cho K-S (2010) Earthworm cast as a promising filter bed material and its methanotrophic contribution to methane removal. J Hazard Mater 176:131–138
Nadalig T, Farhan Ul Haque M, Roselli S, Schaller H, Bringel F, Vuilleumier S (2011) Detection and isolation of chloromethane-degrading bacteria from the Arabidopsis thaliana phyllosphere, and characterization of chloromethane utilization genes. FEMS Microbiol Ecol 77:438–448
Nikiema J, Bibeau L, Lavoie J, Brzezinski R, Vigneux J, Heitz M (2005) Biofiltration of methane: an experimental study. Chem Eng J 113:111–117
Park S, Lee I, Cho C, Sung K (2008) Effects of earthworm cast and powdered activated carbon on methane removal capacity of landfill cover soils. Chemosphere 70:1117–1123
Qiu Q, Conrad R, Lu Y (2009) Cross-feeding of methane carbon among bacteria on rice roots revealed by DNA-stable isotope probing. Environ Microbiol Rep 1:355–361
Rainey FA, Ward-Rainey NL, Janssen PH, Hippe H, Stackebrandt E (1996) Clostridium paradoxum DSM 7308T contains multiple 16S rRNA genes with heterogeneous intervening sequences. Microbiology 142:2087–2095
Rivera-Utrilla J, Bautista-Toledo I, Ferro-García MA, Moreno-Castilla C (2001) Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J Chem Technol Biotechnol 76:1209–1215
Saari A, Martikainen PJ (2003) Dimethyl sulphoxide (DMSO) and dimethyl sulphide (DMS) as inhibitors of methane oxidation in forest soil. Soil Biol Biochem 35:383–389
Scheutz C, Kjeldsen P (2003) Capacity for biodegradation of CFCs and HCFCs in a methane oxidative counter-gradient laboratory system simulating landfill soil covers. Environ Sci Technol 37:5143–5149
Scheutz C, Kjeldsen P (2005) Biodegradation of trace gases in simulated landfill soil cover systems. J Air Waste Manag Assoc 55:878–885
Scheutz C, Kjeldsen P, Bogner JE, De Visscher A, Gebert J, Hilger HA, Huber-Humer M, Spokas K (2009a) Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste Manag Res 27:409–455
Scheutz C, Pedersen GB, Costa G, Kjeldsen P (2009b) Biodegradation of methane and halocarbons in simulated landfill biocover systems containing compost materials. J Environ Qual 38:1363–1371
Semrau JD, DiSpirito AA, Yoon S (2010) Methanotrophs and copper. FEMS Microbiol Rev 34:1–36
Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J (2001) Quantification of expression of Staphylococcus epidermidis housekeeping genes with Taqman quantitative PCR during in vitro growth and under different conditions. J Bacteriol 183:7094–7101
Wagner R (1994) The regulation of ribosomal RNA synthesis and bacterial cell growth. Arch Microbiol 161:100–109
Weinbauer MG, Fritz I, Wenderoth DF, Hofle MG (2002) Simultaneous extraction from bacterioplankton of total RNA and DNA suitable for quantitative structure and function analyses. Appl Environ Microbiol 68:1082–1087
Will C, Thurmer A, Wollherr A, Nacke H, Herold N, Schrumpf M, Gutknecht J, Wubet T, Buscot F, Daniel R (2010) Horizon-specific bacterial community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl Environ Microbiol 76:6751–6759
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NRL program, R0A-2008-000-20044-0), and RP-Grant 2012 of Ewha Womans University.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.G., Moon, KE., Yun, J. et al. Comparison of RNA- and DNA-based bacterial communities in a lab-scale methane-degrading biocover. Appl Microbiol Biotechnol 97, 3171–3181 (2013). https://doi.org/10.1007/s00253-012-4123-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4123-z