Abstract
Solid-state fermentation (SSF) from organic wastes can be considered as a novel biotechnological alternative for the development of new processes that may substitute the current chemical-dependence approaches. In this framework, the production of a bioflocculant through SSF using soybean fiber wastes as the sole substrate with the inoculation of Bacillus subtilis UPMB13 at different fermentation stages were carried out in a pilot scale near-to-adiabatic reactors of 4.5 L volume for 10 days. Samples collected at 2, 4, 7 and 10 days were checked for flocculating activity through kaolin assays and the crude bioflocculant was extracted through ethanol precipitation. The highest activities were measured at the 2nd day of fermentation and maintained until the 4th day with an average value of 88.1–92.0 and 92.4–92.7 % of bioflocculant activity for reactors inoculated at the beginning of SSF and after the thermophilic stage, respectively. The bioflocculant activities decreased around 30–40 % by the 7th day. About 30 mg of crude bioflocculant can be obtained from 20 g of sampled fermented substrate at the maximum flocculating activity measured. This SSF process can be considered as a new green alternative to produce bioflocculant from wastes under easily scalable conditions. Further studies may contribute to the industrial production of bioflocculants from wastes as an alternative to the commonly harmful commercial marketed flocculants.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Highlights
-
Positive production of bioflocculant from soybean wastes.
-
SSF may substitute SmF for bioflocculant production.
-
Bioflocculant production proven feasible in scalable pilot scale.
Introduction
The emerging interest of finding and developing environmentally sound approaches in addressing environmental issues as alternatives to the conventional chemically-centered sector has brought the opportunity of exploring the vast possibilities of wastes use as raw materials sources. In that sense, solid state fermentation (SSF) as a promising green biotechnological process that utilizes wastes as substrates for the production of interesting materials, contributes not only to the production of these compounds, but also focuses on the minimization of wastes disposal. SSF is a fermentation process of humid solid substrate in the absence or near absence of free water (Navarro et al. 2011). In this case, soybean fibers residues were used as the sole substrate for the production of extracellular polymeric substances (EPS) with flocculating capabilities; known as bioflocculants, through SSF with additional inoculation of Bacillus subtilis UPMB13. Bioflocculants are EPS produced naturally from organisms that assist in the process of water clarification that are biodegradable in nature and environmentally safe (Deng et al. 2003). Most reported literature discussed the production of EPS; including bioflocculants, from submerged fermentations (SmF), which are reported to be comparably higher in cost due to the large amount of substrate needed to achieve maximum yield. In comparison, the implementation of SSF seek to overcome this issue by utilizing readily usable substrate that are of no use for other purposes and are available in large amounts (Zhao et al. 2012). This research aimed to determine the possibilities of producing the bioflocculants through SSF by utilizing soybean wastes with added inoculation at a scalable level as a projection for future prospect in a full scale industrial level.
Materials and Methods
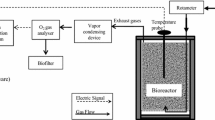
Soybean fibers leftover from the production of tofu were selected as the sole substrate for the solid state fermentation. Addition of wood chips at the ratio of 1:2 to the soya fibers acted as bulking agent which promotes porosity and aeration to the substrate system. The substrate mixture with an initial moisture content of around 60–65 % was then inserted into 4.5 L pilot scale near-to-adiabatic bioreactors at a total mass of around 1,200 g of substrate in each reactor. Continuous oxygen supply was provided all throughout the fermentation at a rate of 0.1 nL/min. Bacillus subtilis UPMB13 strain; primarily researched to be able to produced bioflocculant via submerged fermentations (SmF), was used as the added inoculum at a level of 5 % (v/w) to the system. Two treatments were used, namely, inoculation at initial time (Bioreactor A) and after the thermophilic phase (Bioreactor B). Around 20 g of samples were collected at 2, 4, 7 and 10th day of fermentation for bioflocculant extraction and measurement of flocculating activities. The flocculating activities were measured according to the method described by Zulkeflee et al. (2012) using kaolin as the suspended particles. The bioflocculants were obtained from the fermented substrate at the point of maximum flocculating activity measured, by suspending the sample in 10 volumes of ultra-pure water, shaken on an orbital shaker for 1 h and gone through multiple steps of centrifugation and filtration to separate the residual visible substrate. The resulting filtrate was added to two volumes of ice cold ethanol to precipitate the bioflocculants. Crude solid bioflocculants were obtained through lyophilization.
Results and Discussion
The flocculating measurement reflects the bioflocculant production during fermentation. The results from the kaolin assay are depicted in Fig. 33.1.
Flocculating activity measured from the 20 g of fermented substrate sampled at the specific fermentation time. The symbol  denotes Bioreactor A, where inoculation of Bacillus subtilis UPMB13 was done at initial time while the symbol
denotes Bioreactor A, where inoculation of Bacillus subtilis UPMB13 was done at initial time while the symbol  denotes Bioreactor B, inoculated after the thermophilic phase of the fermentation
denotes Bioreactor B, inoculated after the thermophilic phase of the fermentation
At the initial point of fermentation, it is observed that a slight flocculating activity was measured in both treatments, noted to be considerably low, may be due to the charge destabilization by the ionic components presence in the substrate mixture when tested with the kaolin suspension. As the fermentation continues, the flocculating activities in both reactors increased steeply by the 2nd day and reached a maximum value of around 92 % by the 4th day of fermentation suggesting that the production of bioflocculant occurred in parallel with the strain growth. A decrement of about 30–40 % of flocculating activities can be seen at the 7th day of fermentation until the 10th day, suggesting that the declination of production of the bioflocculant and the loss of the available bioflocculant in the system as a result of prolonged cultivation, could have led to nutrients scarcity and, in consequence, the excreted bioflocculants could be consumed by the microbes as substitute for food (Xia et al. 2008). It is noted that the similar performance and production trend can be seen in both reactors suggesting the presence of indigenous microbial community in the substrate system that are capable of producing the bioflocculant and denoting that there were no direct effects of inoculation by the strain Bacillus subtilis UPMB13.
About 30 mg of crude bioflocculant can be obtained from the 20 g of fermented substrate sampled at the 4th day of fermentation. The value may be an underestimation caused by the potential loss throughout the extraction process.
Conclusion
The study showed promising results on the utilization of soybean fiber residues for the production of bioflocculant at an easily scalable pilot level. Although the effect of inoculation had not yet been proven, the possibilities of further production enhancement by the strain Bacillus subtilis UPMB13 to these SSF setup might be crucial to be explored as it had been observed in another study by the authors that the strain UPMB13 can produce high performing bioflocculant through SmF by using a soy based liquid substrate. Further research should address the optimization setup for the bioflocculant production with or without the strain by identifying key influencing factors highlighting both feasibility and efficiency for possible future projection to be implemented at an industrial scale level.
References
Deng S, Bai R, Hu X, Luo Q (2003) Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl Microbiol Biotechnol 60(5):588–593
Navarro AS, Gea T, Barrena R, Sanchez A (2011) Production of lipases by solid state fermentation using vegetable oil-refining wastes. Biores Technol 102(21):10080–10084
Xia S, Zhang Z, Wang X, Yang A, Chen L, Zhao J, Leonard D, Renault NJ (2008) Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Biores Technol 99(14):6520–6527
Zhao G, Ma F, Wei L, Chua H (2012) Using rice straw fermentation liquor to produce bioflocculants during an anaerobic dry fermentation process. Biores Technol 113:83–88
Zulkeflee Z, Aris AZ, Shamsuddin ZH and Yusoff MK (2012) Cation dependence, pH tolerance, and dosage requirement of a bioflocculant produced by Bacillus spp. UPMB13: flocculation performance optimization through kaolin assays. Sci World J 2012:7 doi:10.1100/2012/49565
Acknowledgments
The authors would like to extend their appreciation to Spanish Ministerio de Economía y Competitividad (Project CTM2012-33663) for their financial support and the European Commission under the Erasmus Mundus Action 2 Plan (MAHEVA Project) for the scholarship awarded.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Singapore
About this paper
Cite this paper
Zulkeflee, Z., Sánchez, A. (2014). Green Biotechnological Approach as an Alternative to Chemical Processes: The Case of Bioflocculant Production through Solid-State Fermentation of Soybean Wastes. In: Aris, A., Tengku Ismail, T., Harun, R., Abdullah, A., Ishak, M. (eds) From Sources to Solution. Springer, Singapore. https://doi.org/10.1007/978-981-4560-70-2_33
Download citation
DOI: https://doi.org/10.1007/978-981-4560-70-2_33
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-4560-69-6
Online ISBN: 978-981-4560-70-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)