Abstract
A laccase stage can be used as a pre-treatment of a standard chemical bleaching sequence to reduce environmental concerns associated to this process. The importance of each independent variable and its influence on the properties of the bleached pulp have been studied in depth in this work, using an adaptive network-based fuzzy inference system (ANFIS) with four independent variables (laccase, buffer, mediator and oxygen) as input. Eucalyptus globulus kraft pulp was biobleached using a laccase from Pycnoporus sanguineus and a natural mediator (acetosyringone). Later, an alkaline extraction and a hydrogen peroxide treatment were applied. Most biobleaching processes showed a decrease in kappa number and an increase in brightness with no significant impact on the viscosity values, compared with the control. Oxygen was the variable with the smallest influence on the final pulp properties while the laccase and buffer solution showed a significant influence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of chlorine and chlorine compounds in the conventional bleaching process leads to the generation of toxic compounds such as chlorolignins. To avoid this environmental hazard, totally chlorine-free (TCF) bleaching processes have been developed which use nonchlorinated chemicals such as oxygen, ozone or hydrogen peroxide. However, as these nonchlorinated agents are not as lignin-selective as chlorine derivates are (such as hypochlorite or chlorine dioxide), they can degrade cellulose, therefore decreasing the pulp yield and rendering a paper with poorer mechanical properties. This is one of the reasons why elemental chlorine-free (ECF) sequences are still frequently used.
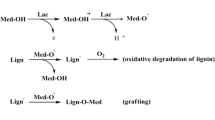
A valuable alternative that helps to address the environmental concerns and the low selectivity which are inherent to the ECF and TCF bleaching sequences, respectively, is the use of microbial enzymes to treat pulps before applying the chemical bleaching sequences. The laccases are among the most useful enzymes applied in biobleaching, as they are capable of oxidizing a wide variety of phenolic compounds [1], requiring only molecular oxygen to complete the process. Moreover, the oxidative ability of laccases can be extended to non-phenolic structures through the action of some low molecular weight compounds, called mediators. Laccases transform these mediators in relatively stable radicals, which continue oxidizing other compounds that are not direct substrates of the enzyme [2]. The use of a laccase and a mediator is commonly known as a laccase-mediator system (LMS). Several authors have shown that pretreating with a LMS reduces the consumption of chemical bleaching reagents in subsequent bleaching stages, does not affect paper quality, and generates less polluted effluents [3–8]. However, the application of LMS has some limitations, such as the high cost of the enzymes and the toxicity of the synthetic mediators, which prevent the industrial full-scale application of LMS in biobleaching sequences. Another drawback concerns the conditions of the enzymatic pre-treatment, which are determined by laccase stability, and differ considerably from the temperature and pH employed in a conventional TCF bleaching sequence. Although many studies have focussed on overcoming these limitations, few of them [6, 9–12] have taken a comprehensive approach to assess all the variables involved in an enzymatic process, individually or in combination, and their influence on the bleached pulp properties.
Consequently, the aim of our work here was to apply a 24 experimental design to determine the degree of influence that the main variables involved in a LMS pretreatment exert on bleached pulp properties. Experiments were performed on an industrial Eucalyptus globulus kraft pulp and the biobleaching sequence consisted of a LMS pre-treatment, an alkaline extraction to remove most of the enzymatically oxidized lignin, and a hydrogen peroxide bleaching stage. To verify if the laccase pre-treatment boosts the global bleaching sequence and to determine which variables of the enzymatic pretreatment influence pulp properties the most, the following pulp features were assessed: kappa number, viscosity, hexenuronic acid (HexA) content, and brightness. The consumption of hydrogen peroxide and the stability of the optical properties of the resulting paper sheets against aging were also evaluated. An adaptive network-based fuzzy inference system (ANFIS) was used to analyze and model the results.
Experimentals
Chemicals
All chemicals were reagent grade. NaOH, MgSO4, and H2O2 were obtained from Panreac Química SAU (Barcelona, España); 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonate (ABTS) was purchased from Roche (Madrid, Spain); diethylene triamine pentaacetic acid (DTPA) was purchased from Merck (Barcelona, Spain), and acetosyringone (4-hydroxy-3,5-dimethoxyacetophenone) was purchased from Sigma–Aldrich (Madrid, Spain).
Raw material
Industrial unbleached E. globulus kraft pulp was provided by La Montañanesa pulp mill (Torraspapel-Lecta Group, Spain). Its Kappa number, brightness, viscosity and hexenuronic acid (HexA) content were 14.2, 32.9 % ISO, 1,257 mL/g, and 39.0 μmol/g, respectively.
Laccase enriched extracellular liquid produced by Pycnoporus sanguineus
A laccase enriched extracellular liquid was used as laccase source in enzymatic pretreatment. This extracellular liquid was produced by P. sanguineus as described elsewhere [13]. Laccase activity in this solution was 1.9 U/ml, measured with the method of Mansur et al. [14] using ABTS as substrate. One laccase unit is defined as the amount of enzyme needed to produce 1 μmol of product per minute in the assayed conditions.
To select the optimum pH and temperature for the enzymatic pretreatment, laccase activity and stability of the extracellular liquid were assessed during 3 h at different pHs (using citrate–phosphate as buffer) and temperatures (25–70 °C). Based on these test results (published in Eugenio et al. [15]), the conditions selected for the enzymatic pretreatment were a pH of 3 and a temperature of 40 °C.
Experimental design to study the enzymatic stage
A 24 factorial design was applied to examine the influence of each independent operational variable (the addition or absence of laccase, buffer, mediator and oxygen) on the characteristics of the bleached pulp (kappa number, viscosity, HexA content, brightness and brightness stability) and on the consumption of chemicals in the hydrogen peroxide stage.
The enzymatic pretreatment (L) was performed introducing 50 g of industrial E. globulus kraft pulp in 500-mL reactors and mixing it with laccase (laccase enriched extracellular liquid), mediator and/or citrate–phosphate buffer (0.1 M). Acetosyringone was the natural mediator added in a concentration of 0.05 mmol/g odp.; and the dose of laccase was 2.4 UA/g o.d.p. In experiments in which oxygen was added, it was introduced in the reactors until reaching a pressure of 6 kg/cm2 before reactors were submerged in a thermostatic bath. Consistency, reaction time, temperature and pH were fixed at 10 %, 1 h, 40 °C and pH 3, respectively, in consistency with previous reports [8]. Also, some drops of 0.05 % Tween 80 were added.
The total number of experiments required for our considered independent variables at two levels was 16. To facilitate direct comparison of the coefficients and visualization of the effects of each individual independent variable on the response variable, the values of the independent variables were normalized from −1 to +1 by using Eq. 1.
where X n is the normalized value of the independent variable; X is the absolute experimental value of the variable concerned; \(\bar{X}\) is the mean of all the experimental values for the variable in question; and X max and X min are, respectively, the maximum and minimum values of the variable.
In our report, +1 and −1 represent presence and absence of laccase, buffer, mediator and oxygen (the independent variables). Therefore, when laccase, buffer, mediator and oxygen are +1, it means that these species are added to the enzymatic bleaching under the following final conditions: 2.4 UA/g o.d.p., 100 mM, 0.05 mmol/g o.d.p. and 6 kg/cm2, respectively.
The enzymatic pre-treatment was followed by an alkaline extraction (E) with 1.5 % NaOH, at 5 % consistency and 90 °C for 120 min. Subsequently, pulps were washed and hydrogen peroxide (P) was applied in a bleaching stage under the following conditions: 1 % H2O2, 1.5 % NaOH, 1 % DTPA, 0.2 % MgSO4, and 5 % consistency and 90 °C for 90 min. Residual hydrogen peroxide was analyzed in the bleaching effluent by standard titration.
All bleached pulps obtained were characterized in terms of their kappa number, brightness and viscosity according to the ISO 302, ISO 2470 and ISO 5351-2 standards, respectively. Additionally, the hexenuronic acid (HexA) content was measured with the method of Gellerstedt and Li [16]. Finally, bleached pulps were subjected to accelerated aging to assess brightness stability. This accelerated aging was carried out in a climatic test cabinet CTS (model C-20/250/S); it consisted in moist heat treatment at 80 °C and 65 % relative humidity for 6 days, following the specifications of the ISO 5630-3 standard.
Statistical analysis by adaptive neural fuzzy inference system (ANFIS)
Fuzzy modeling, based on the pioneering idea of Zadeh [17], is a powerful tool to describe the behavior of non-linear complex systems; and fuzzy theory has been successfully applied to the simulation and control of several biological processes. Another powerful tool for modeling these complex systems is neural networks (NN), which mimic the features of biological neurons. The integration of fuzzy systems and neural networks can combine the merits of both systems, offering a more powerful tool for modeling. This approach uses NN as a tool in fuzzy systems. In that way, the adaptive network-based fuzzy inference system (ANFIS), proposed by Jang [18], is based on the first order Sugeno-fuzzy model. The NN paradigm used is a multilayer feedforward back-propagation network. Details on the architecture and the learning procedure of ANFIS can be found in related literature [18].
The mathematical equation, which responds to different rules, is:
where y e estimate value of output variable, m number of rules, y l defuzzifier. R l is the product of the selected membership functions.
The simplified expression of Eq. 2 for the variables studied and 16 fuzzy rules (two levels for each independent variable) is.
In this study, c 1 is a constant (defuzzifier). Figure 1 shows the ANFIS model structure used for all the independent variables.
Parameters, constants and membership functions in Tables 2 and 3 were determined using the ANFIS tool in the Matlab (Fuzzy Logic Toolbox Version 2.2.2, Neural Network Toolbox Version 4.0.6) software suite.
Determination of mediators in the extracellular liquid
Supernatants from uninoculated control and cultures of P. sanguineus were extracted with water by the following procedure: Supernatants were filtered (Whatman No. 1) and acidified to pH 1–2 with 12 M HCl. After removing precipitates by centrifugation, the concentrations of low molecular weight aromatic compounds were measured in the supernatants. These compounds were extracted with three volumes of peroxide-free diethyl ether. The organic layer was collected, dried over anhydrous Na2SO4, and filtered through Whatman no. 54 filter paper. The residues were dried under a stream of nitrogen gas [19]. Dry residues were dissolved in 25 μL dichloromethane, and 1 μL aliquots were analyzed by (Gas chromatography/Mass spectrometry) GC–MS in an Agilent 6,890 system equipped with a fused silica capillary column HP 5MS (30 m × 250 μm × 0.25 μm inner diameter). The detector was an Agilent 5973 mass selective detector (EI at 70 eV). Oven temperature was held at 50 °C for 1 min before increasing it up to 100 °C at a rate of 30 °C/min; then, from 100 to 300 °C, the rate of temperature rise was 10 °C/min, and the oven was kept isothermal at 300 °C for 10 min using a heating rate of 20 °C/min in scan modus. The carrier gas used was helium with a controlled flow of 1 mL/min. Products were identified based on the Wiley and NIST computer libraries.
Results and discussion
ANFIS model and influence of independent variables
As per the established experimental protocol, 16 experiments consisting of a LMS pretreatment (L), an alkaline extraction (E) and a hydrogen peroxide bleaching stage (P) were carried out. Table 1 shows the normalized values of independent variables, features of the pulp obtained after the LEP biobleaching sequences and hydrogen peroxide consumption. Each value is an average calculated with three samples, the standard deviation being lower than 5 %.
Tables 2 and 3 show the parameters, constants and membership functions obtained introducing the values of the independent variables (in accordance with the predefined experimental design) in the fuzzy models for each measured dependent variable. The ANFIS analysis of each dependent variable as a function of the independent variables yields the mathematical models shown in Table 3. For this study, the optimum number of rules was set to be 16 for all the dependent variables, subsequently developing Eq. 3. Table 3 lists values for kappa number, brightness (% ISO), viscosity (mL/g) and HexA (μmol/g) of LEP bleached pulps, hydrogen peroxide consumption during the P stage and brightness reversion (% ISO) after accelerated aging of bleached pulps. Differences between experimental values and those that were estimated using the previous equations never exceeded 10 % of the former.
For this study, in Eq. 3, c 1 values were parameters estimated to minimize error. In addition, linear membership functions were selected, with 2 functions per independent variable, representing low and high independent variable values. Table 3 shows membership functions for each independent parameter.
Moreover, R i = Π x i, is defined as the product of four membership functions (one for each independent variable), the combinations of membership functions are:
where lacasse (L): 1 (absence), 2 (2.4 U/g o.d.p.); buffer (B): 1 (absence), 2 (100 mM, using citrate–phosphate); mediator (M): 1 (absence), 2 (0.05 mmol/g o.d.p.); and oxygen (O): 1 (absence), 2 (6 kg/cm2).
Consequently, y l represents the linear behavior of the system in the conditions specified by the independent variables that R l defines.
Identifying the independent variables with maximum and minimum influence on the dependent variables can be rather challenging due to the complexity of those terms in the equations that represent interactions between independent variables. Figure 2 shows a way to approach this problem by plotting all dependent variables against each independent variable across the normalized domain, −1 to +1. At every given value of an independent variable X, the magnitude of the difference between the maximum and minimum values of the dependent variable Z is a function of the other independent variables. Thus, if all other independent variables had no effect on Z, the difference between the maximum and minimum values of Z would be zero (and there would be only one point in the graphs of Fig. 2); on the other hand, if the influence of the other independent variables were absolute (i.e. if X had no effect at all on Z), the difference between maximum and minimum Z would be constant for any value of X. In other words, the graph would be a rectangle determined by the domain of values of X, \(\left[ {\left( {X_{ni} } \right)_{\hbox{max} } {-}\;\left( {X_{ni} } \right)_{\hbox{min} } } \right],\) , as the base, and the maximum possible range of Z, \(\left\{ {\left[ {Z\left( {X_{ni} } \right)_{\hbox{max} } } \right]_{\hbox{max} } {-}\,\,Z\left[ {\left( {X_{ni} } \right)_{\hbox{min} } } \right]_{\hbox{min} } } \right\}\) as the altitude.
Because the influence of all the other independent variables on a particular dependent variable can vary with each value of the independent variable that is being considered in the graph, the average change in the dependent variable will be given by:
The change in any given dependent variable with the independent variable considered in the graph can be assimilated to the difference \(\left[ {Z\left( {X_{ni} } \right)_{\hbox{max} } } \right]_{\hbox{max} } {-}Z\left[ {\left( {X_{ni} } \right)_{\hbox{min} } } \right]_{\hbox{min} }\) and the previous expression:
These values make it possible to weigh the relative influence, as a percentage, of each independent variable on the variation of each dependent variable (Fig. 2).
As Fig. 2 shows, lacasse is the most influential variable on kappa and viscosity and buffer (pH adjustment) has the greatest influence on brightness, HexA content, hydrogen peroxide consumption and brightness reversion during aging. Addition of buffer means acidification to a pH of 3, which, based on the study of stability of the P. sanguineus extracellular liquid, results in higher laccase stability and activity [15]. Consequently, addition of laccase and buffer to the enzymatic pretreatment contributed to enhance the bleaching process, increasing delignification and brightness of the bleached pulps, but causing also a slight decrease in viscosity, probably due to degradation of some carbohydrates during delignification in the P stage. Furthermore, lowering the pH enhanced the hydrogen peroxide bleaching stage, reducing hydrogen peroxide consumption. This finding can be explained by a more efficient enzymatic pretreatment and the elimination of metallic ions (such as Fe2+, Mn2+ and Cu2+) in acid pH [20]. In the presence of these metallic ions, hydrogen peroxide could be decomposed forming hydroxyl radicals that would reduce the efficiency of the hydrogen peroxide stage [21], a possibility when buffer is not added. These metallic ions could also decrease brightness as a result of the formation of colored complexes [22], which would account for the high influence of the buffer variable on brightness. Finally, several authors have reported that an acid pH can contribute to the removal of HexA [8, 23], which would reduce also brightness reversion during aging [8, 24]. However, in the present study, HexA content remained fairly consistent (between 29.8 and 34.2 μmol/g of dry pulp) at the end of the bleaching sequence. Moreover, not only HexA content but also lignin and hemicelluloses content can modify the response to accelerated aging [25].
As mentioned above, laccase and buffer are the variables that have the greatest influence on bleachability, which suggests that the laccase enriched extracellular liquid isolated from P. sanguineus, when applied at optimal pH, is able to degrade lignin and enhance the subsequent chemical bleaching, even without the addition of mediator and oxygen. In other words, the relative influence exerted by the other two independent variables (mediator and oxygen) has been found to be minimal. A plausible explanation could be certain substances naturally present in the extracellular liquid which could work as mediator (discussed on Sect. 3.3), somehow reducing the process dependence on the addition of an external mediator [26]. Finally, the presence of dissolved oxygen in the extracellular liquid could be enough to meet the requirements of the enzymatic reaction. Similarly, in their study of flax pulp biobleaching with a commercial laccase and HBT as mediator, Fillat and Roncero [10, 11] have found that 0.2–0.6 MPa of oxygen have no effect on brightness or the kappa number.
Response surfaces for dependent variables
To determine those values of the independent variables that result in optimum kappa number and brightness (the outputs that better reflect the effectiveness of the biobleaching process), the response surfaces for each dependent variable were plotted at two extreme levels of the most influential independent variable (Fig. 3).
Figure 3a shows the response surface for Kappa numbers at the end of the LEP sequence. This response surface shows clearly how the addition of laccase strongly influences delignification during biobleaching. Laccase activity increased with the addition of buffer, which enhanced delignification, as was to be expected. Furthermore, even in the absence of laccase, the acid pH resulting from the addition of buffer also improved delignification during the P stage thanks to the elimination of metallic ions, a process mentioned previously. Finally, the change in the kappa number with the addition of mediator was less than 0.2, which confirms that the effect of this variable on the kappa number is small. The response surface for brightness (Fig. 3b) showed similar results to those of the kappa number: the addition of laccase and buffer enhanced the biobleaching process by increasing brightness, while the addition of an external mediator also improved brightness but to a smaller extent. Therefore, taking all of these results in consideration, it can be concluded that the optimum experiment would be that which included laccase, buffer and mediator (although the mediator has a smaller influence), with no need of adding oxygen. Other authors have also reported on the lack of effect of oxygen on brightness and kappa number [10, 11, 27]. Fillat and Roncero [12] have reported that, if the enzymatic pretreatment of flax pulp is conducted at atmospheric pressure, differences in delignification and brightness between experiments with and without air or oxygen bubbled in the reaction liquid only are observed after the first 3 h of treatment. This report is consistent with our findings after 1 h of enzymatic treatment.
Viscosity is an indirect indicator of the degree of polymerization of cellulose samples [28]. Changes in viscosity give an indication as to carbohydrate breakdown during the biobleaching sequence. All the experiments produced pulps with an adequate viscosity, and no big differences were found between them. However, as Fig. 3c shows, slightly higher viscosities were found when no laccase, buffer or mediator was added. This could be the consequence of the delignification observed in these experiments, since delignification could contribute to the degradation of some carbohydrates bonded to lignin.
No significant changes in HexA content (Fig. 3d) were observed across the different experiments; values were always between 29.8 and 34.2 μmol/g. These results indicate that the LMS consisting of laccase from P. sanguineus and acetosyringone is not able to remove HexA from the pulp under the conditions here used, which is not consistent with what Eugenio et al. [8] have observed. However, other authors have reported no removal of HexA when acetosyringone was combined with laccase from Myceliophthora thermophila [29] or Trametes sp. I-62 [30].
As regards the hydrogen peroxide consumption (Fig. 3e), the most influential variable was buffer. A pH of 3 (addition of buffer) contributes to the removal of metallic ions [20] which, if present, can cause the breakdown, and therefore increased consumption, of hydrogen peroxide [21]. In other words, addition of buffer contributed to reduce hydrogen peroxide consumption. Also observed was that the presence of mediator increased the consumption of hydrogen peroxide, probably as a result of grafting of the mediator (effected by the laccase) into the fibers [31], which would consume part of the bleaching reagent.
After aging of the bleached pulps, the reduction in brightness was found to be independent of the mediator and oxygen presence. Thus, Fig. 3f shows that, when the laccase was most active (addition of laccase and buffer), the reversion of brightness was the highest. Controversial results have been found regarding the effect of LMS on brightness stability. Thus, Martin-Sampedro et al. [29, 32] reported lower stability of optical properties when pulps from EFB (empty fruit bunches) or olive tree pruning were treated with a commercial laccase from Myceliophthora thermophila and acetosyringone, compared with their respective controls. When an industrial eucalyptus pulp was biobleached with the same LMS [27], or with a laccase from Trametes sp. I-62 and acetosyringone [30], the reversion of brightness was also higher than in control assays. Contrarily, other authors have reported improved brightness stability after a LMS treatment on eucalypt pulp, but all of them observed removal of HexA during the enzymatic treatments [8, 24]. Therefore, although the type of enzyme, mediator and pulp influence the stability of the pulp optical properties, and not only the HexA content, but also the lignin and hemicelluloses content can modify the response to accelerated aging [25], results from previous authors seems to indicate that the optical stability is closely related to the removal of HexA during the LMS treatment. Consequently, having observed no significant removal of HexA in the present study, it would appear to be reasonable to expect no improvement in optical stability when the laccase activity increased.
Lastly in our study, to verify the importance of the biological pretreatment, an alkaline extraction and a hydrogen peroxide treatment were applied to the initial industrial kraft pulp without any pretreatment (control experiment). Here, the kappa number was 34.2 % greater and brightness resulted to be 12.2 % ISO lower than values found in the LEP bleached pulp at optimum experimental conditions (in presence of laccase, buffer, mediator and oxygen). Therefore, an LMS pretreatment, only 1 h long, would enhance the bleaching sequence, obtaining similar results to those from longer treatment times, even when higher laccase doses were applied [6, 9, 29, 32–34]. Leaving aside potential differences in laccase–mediator system effectiveness, the reason for this finding, could be that the enzyme might no longer be stable after certain reaction times. Once again, it is worth mentioning that, although a slight reduction in brightness and an increase in kappa number were observed when oxygen was not added in the optimal experiment, no significant influence of oxygen was observed in the bleaching process conducted according to the experimental design of this study. Therefore, it seems within reason to claim that the enzymatic stage can be carried out without adding oxygen, thus reducing the cost of the biobleaching process.
Determination of mediators on the extracellular liquid
According to Figs. 2 and 3, the effect produced on any dependent variable by adding a mediator (acetosyringone) during the enzymatic stage is lower than 19 % in all cases. These results could be explained by the presence of certain substances in the extracellular liquid, which could act as mediator; other authors have found these substances in an extracellular liquid obtained from Trametes trogii used to biobleach loblolly pine kraft pulp [26]. In this instance, these authors regarded the addition of an external mediator unnecessary, and claimed that such an addition even reduced the efficiency of biobleaching. For this reason, the extracellular liquid obtained from P. sanguineus after 13 days of incubation was analyzed in search of natural mediators.
None of the compounds detected in Gas chromatograms/mass spectra (GC–MS) of diethyl ether extracts of the acidified supernatants obtained from cultures of P. sanguineus can be considered laccase substrates; therefore, it may well be assumed that no mediator was produced by this fungus after 13 days of incubation (data not shown). However, before any definite conclusion is drawn in this regard, a complete kinetic study along the time course of growth of the microorganism would be required to screen all kinds of phenolic compounds released in the process. Additionally, cultures should be held in the presence of lignocellulosic materials to trigger the release of phenolic compounds derived from lignin depolymerisation through the action of this ligninolytic fungus [35, 36]. Furthermore, there is a possibility that some phenolic compounds released through delignification during the pulping process could be adsorbed onto the unbleached pulps, and act also as mediator during biobleaching.
Conclusions
The laccase–mediator system formed by acetosyringone as mediator and a laccase enriched P. sanguineus extracellular liquid has proved to be an effective bleaching booster of a Eucalyptus globulus kraft pulp TCF sequence. The statistical treatment of the experimental data led to the conclusion that oxygen was the variable with the smallest influence on the final pulp characteristics, while laccase and buffer solution showed a significant influence. The addition of an external mediator (acetosyringone) produced only relatively minor changes in all dependent variables—less than 19 %, which suggests that there were already mediators naturally present in the media. However, none of the compounds detected by GC–MS in extracellular liquid obtained from P. sanguineus was identified as such natural mediators.
References
Thurston CF (1994) The structure and function of fungal laccases. Microbiol 140(1):19–26
Bourbonnais R, Paice M (1992) Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). Appl Microbiol Biotechnol 36(6):823–827. doi:10.1007/bf00172202
Bajpai P, Anand A, Bajpai PK (2006) Bleaching with lignin-oxidizing enzymes. Biotechnol Annu Rev 12:349–378
Kapoor M, Kapoor R, Kuhad R (2007) Differential and synergistic effects of xylanase and laccase mediator system (LMS) in bleaching of soda and waste pulps. J Appl Microb 103(2):305–317
Moldes D, Díaz M, Tzanov T, Vidal T (2008) Comparative study of the efficiency of synthetic and natural mediators in laccase-assisted bleaching of eucalyptus kraft pulp. Bioresour Technol 99(17):7959–7965
Valls C, Roncero B (2009) Using both xylanase and laccase enzymes for pulp bleaching. Bioresour Technol 100(6):2032–2039
Eugenio ME, Hernández M, Moya R, Martín-Sampedro R, Villar JC, Arias ME (2011) Evaluation of a new laccase produced by Streptomyces ipomoea on biobleaching and ageing of kraft pulps. Bioresources 6(3):3231–3241
Eugenio M, Santos S, Carbajo J, Martín J, Martín-Sampedro R, González A, Villar J (2010) Kraft pulp biobleaching using an extracellular enzymatic fluid produced by Pycnoporus sanguineus. Bioresour Technol 101(6):1866–1870
Moldes D, Vidal T (2008) Laccase–HBT bleaching of eucalyptus kraft pulp: influence of the operating conditions. Bioresour Technol 99(18):8565–8570
Fillat U, Roncero MB (2009) Effect of process parameters in laccase-mediator system delignification of flax pulp: part I Pulp properties. Chem Eng J 152(2):322–329
Fillat U, Roncero MB (2010) Optimization of laccase-mediator system in producing biobleached flax pulp. Bioresour Technol 101(1):181–187
Fillat U, Roncero MB (2010) Flax biobleaching by laccase mediator system at atmospheric pressure. Afinidad 67(548):254–261
Eugenio ME, Carbajo JM, Martin JA, González AE, Villar JC (2009) Laccase production by Pycnoporus sanguineus under different cultural conditions. J Basic Microbiol 49(5):433–440
Mansur M, Arias ME, Copa-Patiño JL, Flärdh M, González AE (2003) The white-rot fungus Pleurotus ostreatus secretes laccase isozymes with different substrate specificities. Mycologia 95(6):1013–1020
Eugenio ME, Carbajo JM, Martín JA, Martín-Sampedro R, González AE, Villar JC (2010) Synergic effect of inductors on laccase production by Pycnoporus sanguineus. Afinidad 67(546):129–135
Gellerstedt G, Li J (1996) An HPLC method for the quantitative determination of hexeneuronic acid groups in chemical pulps. Carbohydr Res 294:41–51
Zadeh LA (1965) Fuzzy sets. Inf Control 8(3):338–353
Jang J-S (1993) ANFIS: adaptive-network-based fuzzy inference system. IEEE Trans Syst Man Cybern 23(3):665–685
Hernández-Coronado M, Hernandez M, Rodriguez J, Arias M (1998) Gas chromatography/mass spectrometry as a suitable alternative technique to evaluate the ability of Streptomyces to degrade lignin from lignocellulosic residues. Rapid Commun Mass Spectrom 12(22):1744–1748
Roncero MB, Queral M, Colom JF, Vidal T (2003) Why acid ph increases the selectivity of the ozone bleaching processes. Ozone Sci Eng 25(6):523–534
Pekarovicova A, Jameel H, Joice TW (1994) TCF bleaching of wheat straw organo cell pulp. Cellul Chem Technol 28:551–561
Yoon B-H, Wang L-J, Kim G-S (1999) Formation of lignin-metal complexes by photo-irradiation and their effect on colour reversion of TMP. J Pulp Pap Sci 25(8):289–293
Valls C, Cadena EM, Blanca Roncero M (2013) Obtaining biobleached eucalyptus cellulose fibres by using various enzyme combinations. Carbohydr Polym 92(1):276–282
Cadena EM, Vidal T, Torres AL (2010) Influence of the hexenuronic acid content on refining and ageing in eucalyptus TCF pulp. Bioresour Technol 101(10):3554–3560
Sevastyanova O, Lindstrom M (2005) Gellerstedt G Influence of a Bleaching Sequence on the Brightness Stability of Eucalyptus Kraft Pulp. 59th Appita Annual Conference and Exhibition: Incorporating the 13th International Symposium on Wood. Fibre and Pulping Chemistry, New Zealand, p 251
Da Re V, Papinutti L, Villalba L, Forchiassin F, Levin L (2008) Preliminary studies on the biobleaching of loblolly pine Kraft pulp with Trametes trogii crude extracts. Enzyme Microb Technol 43(2):164–168
Martín-Sampedro R, Eugenio M, Villar J (2011) Biobleaching of Eucalyptus globulus kraft pulps: comparison between pulps obtained from exploded and non-exploded chips. Bioresour Technol 102:4530–4535
Evans R, Wallis AF (1989) Cellulose molecular weights determined by viscometry. J Appl Polym Sci 37(8):2331–2340
Martín-Sampedro R, Rodríguez A, Ferrer A, García-Fuentevilla LL, Eugenio ME (2012) Biobleaching of pulp from oil palm empty fruit bunches with laccase and xylanase. Bioresour Technol 110:371–378
Martín Sampedro R, Miranda J, Villar JC, Eugenio ME (2013) Laccase from Trametes sp. I-62: production, characterization and application as a new laccase for Eucalyptus globulus kraft pulp biobleaching. Ind Eng Chem Res 52:15533–15540
Barneto AG, Aracri E, Andreu G, Vidal T (2012) Investigating the structure–effect relationships of various natural phenols used as laccase mediators in the biobleaching of kenaf and sisal pulps. Bioresour Technol 112:327–335
Martín-Sampedro R, Rodríguez A, Requejo A, Eugenio ME (2012) Improvement of TCF bleaching of olive tree pruning residue pulp by addition of a laccase and/or xylanase pre-treatment. BioResources 7(2):1488–1503
Camarero S, Ibarra D, Martinez AT, Romero J, Gutiérrez A, del Río JC (2007) Paper pulp delignification using laccase and natural mediators. Enzyme Microb Technol 40(5):1264–1271
Vivekanand V, Dwivedi P, Sharma A, Sabharwal N, Singh R (2008) Enhanced delignification of mixed wood pulp by Aspergillus fumigatus laccase mediator system. World J Microbiol Biotechnol 24(12):2799–2804. doi:10.1007/s11274-008-9809-0
Eggert C, Temp U, Dean JF, Eriksson K-EL (1996) A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett 391(1):144–148
Camarero S, Ibarra D, Martínez MJ, Martínez ÁT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Env Microbiol 71(4):1775–1784
Acknowledgments
The authors wish to thank the Spanish MINECO for funding this study via Project CTQ 2011-28503-C02-01 and Program PTA 2011-4857-I.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Martin-Sampedro, R., Miranda, J., García-Fuentevilla, L.L. et al. Influence of process variables on the properties of laccase biobleached pulps. Bioprocess Biosyst Eng 38, 113–123 (2015). https://doi.org/10.1007/s00449-014-1249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1249-7