Abstract
Next to xylanases, laccases from fungi and alkali-tolerant bacteria are the most important biocatalysts that can be employed for eco-friendly biobleaching of hard and soft wood pulps in the paper industry. Laccases offer a potential alternative to conventional, environmental-polluting chlorine and chlorine-based bleaching and has no reductive effect on the final yield of pulp as compared to hemicellulases (xylanases and mannanases). In the last decade, reports on biobleaching with laccases are based on laboratory observations only. There are several critical challenges before this enzyme can be implemented for pulp bleaching at the industrial scale. This review discusses significant factors like redox potential, laccase mediator system (LMS)—synthetic or natural, pH, temperature, stability of enzyme, unwanted grafting reactions of laccase, and cost-intensive production at large scale which constitute a great hitch for the successful implementation of laccases at industrial level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases are multicopper oxidases found in fungi, bacteria, plants, and insects; they can oxidize a wide array of organic and inorganic substrates in the mild as well as extreme environmental conditions. Due to their clean reaction mechanism having only water as byproduct, they are very attractive as industrial biocatalysts. Laccases contain three types of copper atoms that can be distinguished by UV/visible and electronic paramagnetic resonance (EPR) spectroscopy. On catalytic sites, the type 1 copper catalyzes the electron transfer, type 2 activates the molecular oxygen, and type 3 is responsible for oxygen uptake. Enzymes lacking the Cu atom responsible for the blue color are called “yellow” or “white” laccases, but widely considered, they are not true laccases (Yaropolov et al. 1994; Morozova et al. 2007; Shleev et al. 2007). Due to continuous criticism and pressure rising by several environmental protection agencies to cease or reduce the direct or indirect involvement of chlorine or chlorine-based chemicals in pulp and paper industries, research efforts have been accelerated worldwide for replacing chemical pulp bleaching technologies with green technologies or bio-based alternatives. One of them is to uphold discoveries of novel pulp delignification enzymes produced by fungi and bacteria. Generally, enzyme-based delignification of pulps occurs below 100 °C and atmospheric pressure. But their proteineous natures, like all enzymes, are proteins that are often sensitive to extreme pH and temperature which influences their three-dimensional structure (Call and Mucke 1997). These biocatalysts cause no deteriorating impact on ground water reservoirs and no risk of soil pollution (Singh et al. 2007, 2011a, b; Virk et al. 2012; Niels and Kepp 2013). The potential of giving enzymatic treatments to pulp was realized in the mid-1980s, when it was discovered that xylanase pretreatments of pulps prior to their subsequent bleaching with chlorine, chlorine dioxide, and hydrogen peroxide could yield substantial reduction in bleaching chemicals (Viikari et al. 1986). Later on, use of lignin degrading enzymes, exclusively the laccases, has been shown to be more effective than xylanases at laboratory or pilot scale studies. Inclusion of laccase before chemical bleaching could reduce the use of chlorine-based chemicals in paper industries (Singh et al. 2011a, b; Virk et al. 2012). Less use of bleaching chemicals means less need of fresh water to wash chemicals from the bleached pulp. The paper industry consumes about 300 m3 of water per ton of paper produced and generates a huge volume of highly colored and toxic carcinogenic effluents (Buzzini and Pires 2007). Initially, the use of laccase for bleaching of hard wood pulps had limited acceptance due to minimal delignification that could be achieved. This inefficiency was attributed to less redox potential and size of the enzyme made it unable to diffuse into pulp fibers to catalyze the oxidation of lignin. Fortunately, this problem was circumvented when Bourbonnais and Paice (1990) discovered that laccase in presence of 2,2ʹ-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) could delignify the kraft pulp. Since then, various research groups have been focusing their attention in search of cost-effective laccase mediator (synthetic or natural) system (LMS) for increasing redox potential of enzyme. Except for a very few alkali-tolerant laccases (Singh et al. 2008; Eugenio et al. 2011), the rest of the biobleaching studies with these enzymes are based on acidic or neutral pH (3.0–6.0). Pulp and paper industries look for highly alkali-tolerant laccases for delignification purposes, because several steps of paper making pass through the alkaline environmental conditions (Singh et al. 2008, 2011a, b). The next hurdle for laccases before bleaching at industrial scale is that they need high oxygen pressure for efficient functioning. On the other hand, majority of laccases have less specific activities; as a result, excessive enzyme units are required before implementing at large-scale biobleaching projects. Less thermo stability of laccases is also a discouraging factor for successful implementation of enzyme at industrial level. Recently, grafting of biobleached pulp was observed by many workers when reaction mixture contained natural mediator and laccase. Although grafting provides strength to pulp fibers but increases the kappa number (KN) and reducing the brightness. Search for the cost-effective methods of enzyme production has always been an important area of enzyme technology. Conventional methods of enzyme production are not appropriate for the large-scale production of laccases from fungi or prokaryotes. Less production of enzyme is a big challenge to continuous supply of laccases to pulp and paper industries at large scale. This review is the first representation of consistent difficulties in the path of successful implementation of laccases at industrial scale for eco-friendly biobleaching of pulps.
Low redox potential (E0) of laccases, inappropriate for proper delignification of pulp
Copper-containing oxidases can be divided into three groups: high, medium, and low redox potential enzymes. Low potential laccases have E0 below 430 mV, medium potential ones have 470–710 mV, and higher potential means an E0 above 710 mV (Morozova et al. 2007). Laccases can only oxidize those compounds whose ionization potential (energy required for removing an electron from its atom) do not exceed the redox potential of T1 copper center. Laccase substrates include aromatic compounds. Many of these compounds are phenolic fragments of lignin. The main purpose of laccases in nature is lignin polymerization and depolymerization (Arora and Sharma 2010). It is believed that for effective delignification of pulp, laccase should be cleaving the non-phenolic lignin structures that depend on the redox potential of the T1 center. The redox potential of non-phenolic lignin structures, such as veratryl alcohol, 1,2-dimethoxybenzene and others are high (>1400 mV). However, it has been demonstrated that laccase is able to oxidize some compounds (redox mediators) with a higher redox potential than laccase itself, although the mechanism by which this happens is unclear. In the presence of such redox mediators, laccase is able to oxidize non-phenolic lignin model compounds and decrease pulp kappa number to a great extent. The standard redox potential range for laccase activity is usually between 500–800 mV versus normal hydrogen electrode (NHE) range which can attack only phenolic moieties comprising less than 10 % of the total polymer in natural lignin (Martínez et al. 2009). Plentiful non-phenolic units in lignin, have redox potential above 1300 mV. The reported redox potentials of laccases are lower than those of non-phenolic compounds (Table 1), so these enzymes cannot oxidize such rigid substances. This limits the range of substrates that can be oxidized by laccases and consequently constrains the delignification of pulp. The E0 of mediators play negligible role in the catalytic efficiency of lignin oxidation; their effectiveness is likely to depend on the chemical reactivity of the radical formed after their initial step of oxidation (Arzola 2009). The possibility to expand their range of oxidation through redox mediators offers considerable biotechnological potential for biobleaching of pulps. The use of such compounds has particular merit because once they are oxidized by laccases to stable radicals, these radicals may continue oxidizing other compounds, including those not used directly as substrates of the enzyme.
Dependency of laccases on mediators for delignification of pulp is cost intensive
Some laccases have higher molecular weight and cannot penetrate deep into the pulp fibers; moreover, due to their rather low redox potential, they are unable to oxidize non-phenolic lignin units. Due to of these limitations, laccase alone can oxidize only phenolic lignin units (<20 % of lignin) on the substrate surface. Therefore, laccases are often applied with an oxidant mediator for biobleaching purposes. A mediator could be a small molecule that acts as an “electron shuttle” between enzyme and substrate. Since the use of ABTS as a mediator for biobleaching of pulp is considered as cost intensive (Singh et al. 2011a, b) at the industrial scale and hydroxybenzotriazole (HBT) has also been shown to inactivate laccases after some time and possesses high toxicity even at low concentrations (Ibarra et al. 2006; Medina et al. 2013), therefore, an extensive search is required to find out cost effective and non toxic natural mediators (Camarero et al. 2007; Reiss et al. 2013). It has been reported that the treatment of different kinds of pulps with Lignozyme—laccase mediator system (LMS) could reduce the kappa number by 70 %. Lignozyme introduced a new mediator, N-hydroxyacetinalide (NHAA) that is biodegradable and has been claimed to be cost effective. Biobleaching with NHAA allows the laccase to maintain about 80 % of its activity after 1-h treatment, but HBT causes a severe loss of enzyme activity. A delignification of >40 % can be obtained with most of the pulps by using 5 kg t−1 HBT, in many cases a dosage of 2.5 kg t−1 HBT pulp was sufficient (Call and Mucke 1995, 1997). Actually, natural mediators are phenols which can be easily extracted from pulping liquors (Camarero et al. 2007; Gutiérrez et al. 2007), effluent streams (Ismail et al. 2005), and plant materials. Natural mediators have been found to perform similarly or even better than synthetic mediators, with increased activity and more modest useful rates (Canas and Camarero 2010). They do not cause inactivation of the enzyme and represent the promising alternative for environmentally friendly delignification of pulps. The first evidence of natural phenols (syringaldehyde (SA) and acetosyringone (AS)) to mediate delignification of eucalypt pulp by laccase from Pycnoporus cinnabarinus at pH 4.0 and 50 ○C (Camarero et al. 2007). When sinapic acid, ferulic acid, coniferyl aldehyde, and sinapyl aldehyde were evaluated as laccase mediators, they lead to lower bleaching efficiency for sisal pulp as these phenolic compounds tend to bind to pulp fibers (Aracri et al. 2009). Fillat et al. (2010) evaluated SA, AS, and p-coumaric acid (PCA) as natural mediators for laccase from P. cinnabarinus at pH 4.0, 50 ○C to bleach flax fibers. Efficiency of these three was compared to HBT in terms of laccase stability. HBT and PCA were found to inactivate laccase in absence of pulp. All natural mediators resulted in a reduced KN after the subsequent alkaline treatment with hydrogen peroxide. Generally, natural mediators increased KN, decreased brightness, and changed optical properties of the pulp after the L stage, suggesting that natural mediators tend to couple to fibers during a laccase mediator treatment (Andreu and Vidal 2011). Therefore, the search for new and more effective mediators involved in natural lignin degradation is needed. In addition, from the point of view of industrial and environmental application, laccase mediators should be environmental-friendly and available at low cost.
Grafting effect of natural mediators on pulp fibers is disappointing for biobleaching
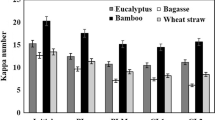
During laccase-based biobleaching of pulps in the presence of natural mediators, the delignification process is hindered by adverse reactions, and then it’s called grafting. The phenoxy derivatives besides lignin oxidation react in several different ways (Fig. 1). Although some authors have emphasized that laccase-catalyzed grafting of phenols onto lignocellulose fibers is an advantageous phenomena, because of their interest in this area, like production of lignin-rich fibers (Aracri et al. 2009). Natural phenols polymerize to yield oligomers or undergo grafting reactions via radical coupling to the pulp surface. Increase in the KN after grafting makes subsequent alkaline hydrogen peroxide extraction mandatory for increasing the brightness of pulp. Barneto et al. (2012) investigated the structural relationship of various natural phenols in biobleaching of kenaf and sisal pulp. It was found that grafting increased the KN of pulp. The mediator that yielded least stable radicals (p-coumaric acid) was the best to graft onto pulp fibers. Most reactive radicals (lacking methoxy groups at ortho-position) show the greatest tendency to collapse with lignin and the least tendency to collapse with other mediator molecules (polymerization). It was concluded that phenolic mediators bearing methoxy groups in ortho-positions show the lowest tendency to undergo grafting reactions and the highest to undergo polymerization. These reactions compete with delignification, representing an adverse and undesirable phenomenon in biobleaching process. At mill scale, biobleaching with natural mediators will not be considered as cost-effective, until an extra step of washing with H2O2 or NaOH is added for increasing the brightness of pulp.
Reaction mechanism of natural phenolic mediators and laccase as a result appearance of grafting of pulp fibers (Barneto et al. 2012)
Acidic working pH of laccases is not appreciable for biobleaching at industrial scale
Pulping is an initial process of paper making, which involves cooking of lignin containing raw material in the presence of NaOH at high temperature and pressure (pH >12 and cooking times 0.5–3 h at temperature 160–180 °C). During pulping, excess of the lignin is removed from the pulp, but still, some embedded intact lignin needs later chemical or enzyme bleaching. After pulping, the pH of pulp is around 9–12 and needs washing with water for reduction in pH. Inclusion of acidic laccases for delignification after pulping stage will add an extra step of reducing pH of pulp; as a result, increase in time and cost of the biobleaching process. Therefore, isolation of novel alkali tolerant laccase for application in biobleaching of pulp is required. Most of the fungal laccases have acidic (3.6 to 5.2) pH optima, while plant laccases works best between 6.8 and 7.4 pH (Dwivedi et al. 2011; Pezzella et al. 2013). Some bacterial laccases showed alkaline pH optima for biobleaching of pulp and decolorization of dyes (Singh et al. 2008; Virk et al. 2013). The pH optima of laccases depend on the nature of substrate (Singh et al. 2007). This variation may be due to changes in the reaction caused by the substrate, oxygen, or the enzyme itself (Kunamneni et al. 2007). Streptomyces cyaneus CECT3335 laccase was reported for biobleaching of eucalyptus kraft pulp at pH 5.0 with ABTS as mediator (Arias et al. 2003). Aracri et al. (2009) delignified sisal pulp at pH 4.0 using laccase from Trametes villosa by trying different mediators (viz. sinapic acid, ferulic acid, coniferyl aldehyde, and sinapyl aldehyde). Eugenio et al. (2010), optimized pH 3.0 for the biobleaching of kraft pulp by using laccase from Pycnoporus sanguineus with acetosyringone as a mediator. Laccase from γ-proteobacterium JB was the first report on biobleaching of agro-based wheat straw-rich soda pulp with alkaline pH 8.0, ABTS as a mediator. The organism grew well from pH 6 to 10 and produced laccase maximally at pH 10 (Bains et al. 2003; Singh et al. 2007, 2008, 2009a). Optimum pH 10 was reported for the modification of alkaline lignin by laccase from entophytic fungus, Mycelia sterilia YY-5 (Weihua and Hongzhang 2008). Moreover, laccases have an advantage over other lignin oxidizing enzymes, like lignin peroxidases (Lip). Redox potential of Lip increases with decrease in pH of reaction environment. For laccases, such data is not available; as logically, alkali-tolerant laccases will be the best choice for pulp bleaching where as alkaline conditions are demanded at large (Singh et al. 2009b; Canas and Camarero 2010).
Low thermostability of laccases is not a favorable factor for biobleaching of pulps
Thermostability of enzymes is an attractive feature for their biotechnological applications (Berka et al. 1997; Singh et al. 2010, 2011a). Laccases are viewed as moderately thermostable and considerable emphasis has been directed for the isolation of increasingly thermostable varieties. Kiiskinen et al. (2004) isolated novel laccases from wood rotting fungi, most of them had t1/2 at 60 ○C of 3–6 h, but pH optima were 2.0–4.0. Reiss et al. (2011) reported laccase from Bacillus pumilus that lost 50 % activity after 1 h at 65 ○C, pH (5–7). Laccase from the spores of Bacillus vallismortis retained more than 50 % activity after 10 h at 70 °C and demonstrated broad pH stability in both acidic and alkaline conditions (Zhang et al. 2013). Weihua and Hongzhang (2008) reported laccase activity was enhanced with increasing reaction temperature with syringaldazine and reached a maximum at 60 °C, but thereafter, it dropped rapidly at higher temperature. The fungal laccases generally have lower thermal stability than bacterial laccases (Hildén et al. 2007; Singh et al. 2011a, b). More thermostable laccases can reduce the reaction time for biobleaching at industrial scale.
Specific activity of laccases and enzyme dosage are crucial aspects for biobleaching
The most striking characteristic of enzymes is their specific activity, that increases as the biocatalyst preparation becomes more pure, since the amount of protein (mg) is typically less, but the rate of reaction remains the same or more due to less interference or removal of inhibitors. Pulp and paper industries are ambitious for high-performance laccases having high specific activity which in turn will be beneficial, because lower enzyme dose will be required for delignification of pulp. Several researchers have optimized the laccase dose in order to use the least amount of enzyme for maximum biobleaching of raw pulp. Studies have revealed that optimum enzyme dose as 10–20 Ug−1 pulp for the laccase-mediated biobleaching (Arias et al. 2003; Camarero et al. 2004; Singh et al. 2008; Aracri et al. 2009; Fillat et al. 2010; Babot et al. 2011). However, for laccase from Streptomyces, an enzyme dose of 2.4 Ug−1 pulp was considered optimum for the delignification of kraft pulp in the presence of acetosyringone as mediator (Eugenio et al. 2011). According to recent studies (Dedhia et al. 2014), 22 Ug−1 pulp laccase dose was optimum for delignification of wheat straw pulp at pH 3.5, 60 °C with HBT as a mediator. Table 2, shows that laccase dose optimized by majority of researchers for biobleaching of pulp is between 10–20 Ug−1 of pulp. This indicates that for biobleaching of 1.0 ton of pulp, industry will need of 10 to 20 millions of laccase units. Requirement of such huge units of laccases can be reduced by dedicated efforts to isolate such microbes that can produce laccases with high specific activity.
Presence of oxygen is an essential factor for biobleaching of pulp
Laccases need O2 to oxidize the lignin components embedded in pulp, by the reduction of one O2 molecule to give one molecule of water plus the oxidized form of the substrate (Yaropolov et al. 1994; Madhavi and Lele 2009; Eugenio et al. 2011). Bourbonnais and Paice (1996) reported ∼32 % delignification (after alkaline extraction stage) when kraft pulp was reacted with laccase from Trametes versicolor in the presence of ABTS under 100–400 KPa of O2 for 2 h. Moldes et al. (2008) reported that O2 pressure was a significant factor for the biobleaching of eucalyptus kraft pulp with laccase from Trametes villosa at pH 4.0, 50 °C. O2 pressure of 600 KPa brought 3.0 to 4.0 % improvement in brightness in all bleaching sequences. Balakshin et al. (2001) have found that most dioxygen takes part in side reactions, even after laccase has lost all activity and only marginally in delignification reactions. The improved pulp properties obtained at a high O2 pressure may have resulted from the delignifying effect of O2 itself. Fillat and Roncero (2009) reported that, the presence of increased amounts of O2 (∼4.0–7.0 ppm) during laccase mediator treatment resulted in more efficient oxidation of lignin of flax pulp and hence, decreased in KN. The presence of increased amount of O2 in the medium enhances the ability of LMS to modify the embedded lignin in pulp to a greater extent than it increases the ability of the enzyme treatment to remove the polymer.
Recombinant laccases in biobleaching of pulps
There is keen research and development (R&D) interests for bringing advancement in application of laccases in the pulp and paper industry. This is demonstrated by a high number of published scientific papers and patents on this topic that continued to increase every year, since 1995. On the other hand, development of laccases for pulp and paper sector is greatly concentrated by biotech companies like Novo Nordisk and Novozymes. Data from the annual report demonstrated that this company has been consistently increasing the investments in R&D and increasing its sales in enzymes for the pulp and paper industry (Demuner et al. 2011). The catalytic improvements of laccases for the better pulp delignification have been tried by the use of some modern biotechnological techniques such as recombinant DNA, mutagenesis, PCR, and encoding. The Aspergillus oryzae laccase producing strain was developed by transformation of A. oryzae host strain How B711 (derived from the A 1560 strain) with two plasmids pRaMB17.WT and pToC90. The pRaMB17.WT plasmid contains the laccase gene from a thermophilic fungus Myceliophthora thermophila that occurs in decaying manure and other organic matter. The laccase gene is linked to the DNA regulatory sequences, promoter, and terminator. The laccase preparation is marketed under a trade name “Flavourstar.” Sigoillot et al. (2004) investigated the pulp bleaching efficiency of P. cinnabarinus laccase expressed in two Aspergillus hosts (A. oryzae and Aspergillus niger), and comparing with the native enzyme. Laccase from wild P. cinnabarinus and A. niger with HBT as redox mediator achieved a delignification up to 75 %, whereas the recombinant laccase from A. oryzae was not able to delignify the pulp. Three laccases were different and each fungal strain introduced differences during protein processing (folding and/or glycosylation). In order to improve laccase treatments of pulp, Ravalson et al. (2009) synthesized a chimeric laccase by fusing P. cinnabarinus laccase lac1 to the carbohydrate-binding module (CBM) of A. niger cellobiohydrolase B. The chimeric protein was investigated for its softwood kraft pulp biobleaching potential in comparison with the native counterpart. By conferring to the chimeric protein the ability to bind to a cellulosic substrate, CBM addition greatly improved laccase delignification properties. The recombinant laccase from Thermus thermophilus was applied to the biobleaching of wheat straw pulp. Optimized conditions for biobleaching were 3.0 U laccase g−1 dry pulp at 90 ○C, pH 4.5, 8 % consistency for 1.5 h. Pulp brightness was increased by 3.3 % ISO, and the pulp kappa number was decreased by 5.6 U. Pulp biobleaching in the presence of 5 mM ABTS further increased the pulp brightness by 1.5 % ISO (Zheng et al. 2012).
Cost-effective production is a challenge for continuous supply of laccase to pulp and paper industries
Madhavi and Lele (2006) reported the highest (692 U ml−1) laccase production from newly isolated fungus WR-1, in the presence of 0.8 mM 2,5-xylidine as an inducer in optimized growth medium. Niladevi et al. (2007) suggested that response surface methodology (RSM) could be used as a valuable statistical method for the optimization of enzyme production from Streptomyces psammoticus; but using this strategy, only threefold enzyme productions was enhanced in comparison to conventional method of enzyme production. Singh et al. (2008) reported the biobleaching of wheat straw-rich soda pulp by alkali-tolerant laccase from γ-proteobacterium JB. Production of this laccase was low (8 × 103 nkat L−1) with conventional method of enzyme production (OVAT system). Later, RSM-based optimization of enzyme production increased laccase production up to 9.3-fold (Singh et al. 2009c). There are several reports of laccase production enhancements available in literature from fungi, but still favorable results not forthcoming according to the pulp and paper industries requirements. Mate et al. (2010) carried out the directed evolution of a laccase gene from basidiomycete PM1, expressed in Saccharomyces cerevisiae. After replacing the native signal sequence with α-factor prepro–leader to regulate the heterologous protein, the fusion protein was subjected to eight rounds of laboratory evolution in combination with rational approaches. The last mutant OB1 showed 34,000-fold enhancement in laccase activity.
Conclusion
Although, several reports are available on laccase-based biobleaching of pulps, still much remains to be discovered and learned about these biocatalysts (Singh et al. 2011a, b; Virk et al. 2013), like clarity about chemistry and mode of action of LMS for efficient and cost-effective delignification of pulps. There is a need to isolate such organisms which can produce alkalophilic, thermostable laccases, possessing high redox potential and specific activity. Sincere efforts are needed to overcome the limitations like low production and cost of mediators that hamper the industrial implementation of laccases.
References
Andreu G, Vidal T (2011) Effects of natural laccases mediator systems on kenaf pulp. Bioresour Technol 102(10):5932–5937
Aracri E, Colom JF, Vidal T (2009) Applications of laccase natural mediators system to sisal pulp: an effective approach to biobleaching or functionalizing pulp fibres? Bioresour Technol 100:5911–5916
Arias ME, Arenas M, Rodrıguez J, Soliveri J, Ball AS, Hernandez M (2003) Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl Environ Microbiol 69:1953–1958
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Arzola GK (2009) Catalytic efficiency of natural and synthetic compounds used as laccase-mediators in oxidising veratryl alcohol and a kraft lignin, estimated by electrochemical analysis. Electrochim Acta 54:2621–2629
Babot ED, Rico A, Rencoret J, Kalum L, Lund H, Romero J, del Río JC, Martínez AT, Gutiérrez A (2011) Towards industrially feasible delignification and pitch removal by treating paper pulp with Myceliophthora thermophila laccase and a phenolic mediator. Bioresour Technol 102:6717–6722
Bains J, Capalash N, Sharma P (2003) Laccase from a non-melanogenic, alkalotolerant γ-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol Lett 25:1155–1159
Balakshin M, Chen CL, Gratzl JS, Kirkman AG, Jakob H (2001) Biobleaching of pulp with dioxygen in laccase–mediator system-effect of variables on the reaction kinetics. J Mol Catal B Enzym 16:205–215
Barneto AG, Aracri E, Andreu G, Vidal T (2012) Investigating the structure effect relationships of various natural phenols used as laccase mediators in the biobleaching of kenaf and sisal pulps. Bioresour Technol 112:327–335
Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, Halkier T, Mondorf K, Xu F (1997) Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol 63:3151–3157
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates. An expanded role for role of laccase in lignin biodegradation. FEBS Lett 267:99–102
Bourbonnais R, Paice MG (1996) Enzymatic delignification of kraft pulp using laccase and a mediator. TAPPI J 79:199–204
Buzzini AP, Pires EC (2007) Evaluation of an up flow anaerobic sludge blanket reactor with partial recirculation of effluent used to treat wastewaters from pulp and paper plants. Bioresour Technol 98:1838–1848
Call HP, Mucke I (1995) Further improvements of laccase-mediator-system (LMS) for enzymatic delignification and results from large scale trials. Int non-chlorine bleaching conf., Amelia Island
Call HP, Mucke I (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym-process). J Biotechnol 53:163–202
Camarero S, Garcıa O, Vidal T, Colom J, del Rıo JC, Gutiérrez A, Gras JM, Monje R, Martınez MJ, Martınez AT (2004) Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzym Microb Technol 35:113–120
Camarero S, Ibarra D, Martinez AT, Romero J, Gutierrez A, del Rio JC (2007) Paper pulp delignification using laccase and natural mediators. Enzym Microb Technol 40:1264–1271
Canas A, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Dedhia BS, Vetal MD, Rathod VK, Levente C (2014) Xylanases and laccase aided bio-bleaching of wheat straw pulp. Can J Chem Eng 92:131–138
Demuner BJ, Junior NP, Adelaide MS (2011) Technology prospecting on enzymes for the pulp and paper industry. J Technol Manag Innov 6(3):49–58
Dwivedi U, Singh P, Pandey V, Kumar A (2011) Laccases from new fungal sources and its promising application. J Mol Catal B Enzym 68:117–128
Eugenio ME, Santos SM, Carbajo JM, Martín JA, Martín-Sampedro R, González AE, Villar JC (2010) Kraft pulp biobleaching using an extracellular enzymatic fluid produced by Pycnoporus sanguineus. Bioresour Technol 101:1866–1870
Eugenio ME, Hernandez M, Moya R, Martin-Stampedro R, Villar JC, Arias ME (2011) Evaluation of new laccase produced by Streptomyces ipomea on biobleaching and ageing of kraft pulps. Bioresources 6:3231–3241
Fillat U, Roncero MB (2009) Biobleaching of high quality pulps with laccase mediator system: influence of treatment time and oxygen supply. Biochem Eng J 44(2–3):193–198
Fillat A, Colom JF, Vidal T (2010) A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour Technol 101:4104–4110
Gutiérrez A, Rencoret J, Ibarra D, Molina S, Camarero S, Romero J, Del Río JC, Martínez AT (2007) Removal of lipophilic extractives from paper pulp by laccase and lignin-derived phenols as natural mediators. Environ Sci Technol 41:4124–4129
Hildén K, Hakala T, Maijala P, Lundell T, Hatakka A (2007) Novel thermotolerant laccases produced by the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol 77:301–309
Ibarra D, Camarero S, Romero J, Martínez MJ, Martínez AT (2006) Integrating laccase-mediator treatment into an industrial-type sequence for totally chlorine free bleaching eucalypt kraft pulp. J Chem Technol Biotechnol 81:1159–1165
Ismail F, Mulholland DA, Marsh JJ (2005) An analysis of water soluble components of Sappi Saiccor’s effluent streams. Water SA 31:569–574
Johnson DL, Thompson JL, Brinkmann SM, Schuller KA, Martin LL (2003) Electrochemical characterization of purified Rhus vernicifera laccase: voltammetric evidence for a sequential four-electron transfer. Biochem 42:10229–10237
Kiiskinen LL, Ratto M, Kruus K (2004) Screening for novel laccase producing microbes. J Appl Microbiol 97:640–645
Klonowska A, Gaudin C, Fournel A, Asso M, Le Petit J, Giorgi M (2002) Characterization of a low redox potential laccase from the basidiomycete C30. Eur J Biochem 269:6119–6125
Kojima Y, Tsukuda Y, Kawai Y, Tsukamoto A, Sugiura J, Sakaino M (1990) Cloning, sequence analysis, and expression of lignolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem 65(25):15224–15230
Kunamneni A, Ballesteros A, Plou FG, Alcalde M (2007) Fungal laccase—a versatile enzyme for biotechnological applications. Commun Curr Res Educ Top Trends Appl Microbiol 233–245
Madhavi S, Lele RSS (2006) Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem 41:581–588
Madhavi S, Lele RSS (2009) Laccase: properties and applications. Bioresources 4(4):1694–1717
Martinez AT, Ruiz-Duenas FJ, Martinez MJ, Del Rio JC, Gutierrez A (2009) Enzymatic delignification of plant cell wall: from nature to mill. Curr Opin Biotechnol 20:348–357
Mate D, Garcia-Burgos C, Garcia-Ruiz E, Ballesteros AO, Camarero S, Alcalde M (2010) Laboratory evolution of high-redox potential laccases. Chem Biol 17(9):1030–1041
Medinaa F, Aguilab S, Barattoc MC, Martoranac A, Basosic R, Alderetea JB, Duhalt RV (2013) Prediction model based on decision tree analysis for laccase mediators. Enzym Microb Technol 52:68–76
Moldes D, Díaz M, Tzanov T, Vidal T (2008) Comparative study of the efficiency of synthetic and natural mediators in laccase-assisted bleaching of eucalyptus kraft pulp. Bioresour Technol 99:7959–7965
Moldes D, Cadena EM, Vidal T (2010) Biobleaching of eucalypt kraft pulp with a two laccase-mediator stages sequence. Bioresour Technol 101:6924–6929
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Biotechnol 43:523–535
Niels JC, Kepp KP (2013) Stability mechanisms of a thermophilic laccase probed by molecular dynamics. PLoS ONE 8(4):e61985. doi:10.1371/journal.pone.0061985
Niladevi KN, Sukumaran RK, Prema P (2007) Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J Ind Microbiol Biotechnol 34:665–674
Oudia A, Queiroz J, Simões R (2008) Potential and limitation of Trametes versicolor laccase on biodegradation of eucalyptus globulus and pinus pinaster kraft pulp. Enzym Microb Technol 43:144–148
Pezzella C, Lettera V, Piscitelli A, Giardina P, Sannia G (2013) Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl Microbiol Biotechnol 97:705–717
Ravalason H, Herpoël-Gimbert I, Record E, Bertaud F, Grisel S, de Weert S (2009) Fusion of a family 1 carbohydrate binding module of Aspergillus niger to the Pycnoporus cinnabarinus laccase for efficient softwood kraft pulp biobleaching. J Biotechnol 142:220–226
Reinhammar BRM (1972) Oxidation–reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim Biophys Acta 275:245–259
Reiss R, Ihssen J, Thöny-Meyer L (2011) Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 11:9
Reiss R, Ihssen J, Richter M, Eichhorn E, Schilling B, Thöny-Meyer L (2013) Laccase versus laccase-like multi-copper oxidase: a comparative study of similar enzymes with diverse substrate spectra. PLos ONE 8(6):e65633
Sadhasivam S, Savitha S, Swaminathan K, Lin F-H (2008) Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Process Biochem 43:736–742
Schneider P, Caspersen MB, Mondorf K, Halkier T, Skov LK, Ostergaard PR (1999) Characterization of a Coprinus cinereus laccase. Enzym Microb Technol 125:502–508
Shleev SV, Morozova OV, Nikitina VO, Gorshina ES, Rusinova TV, Serezhenkov VA, Burbaev DS, Gazaryan IG, Yaropolov AI (2004) Biochimie 86:693–703
Shleev SV, Nikitina OV, Christenson A, Reimann CT, Yaropolov AI, Ruzgas T, Gorton L (2007) Characterization of two new multiforms of Trametes pubescens laccase. Bioorg Chem 35:35–49
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, van den Hondel CAMJJ, Fournel A, Sigoillot JC, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Singh G, Goel R, Capalash N, Sharma P (2007) A pH-stable laccase from Alkali-tolerant γ-proteobacterium JB: purification, characterization and indigo carmine degradation. Enzym Microb Technol 41:794–799
Singh G, Ahuja N, Batish M, Capalash N, Sharma P (2008) Biobleaching of wheat straw-rich-soda pulp with alkalophilic laccase from γ-proteobacterium JB: Optimization of process parameters using response surface methodology. Bioresour Technol 99:7472–7479
Singh G, Batish M, Sharma P, Capalash N (2009a) Xenobiotics enhance laccase production in alkali-tolerant γ -proteobacterium. JB. Braz J Microbiol 40:26–30
Singh G, Capalash N, Sharma P (2009b) Performance of an alkalophilic and halo tolerant laccase from γ-proteobacterium JB in the presence of industrial pollutants. J Gen Appl Microbiol 55:283–289
Singh G, Ahuja N, Capalash N, Sharma P (2009c) Design of experiment methodology for the optimized production of alkalophilic laccase from γ-proteobacterium. JB. Bioresources 2:544–553
Singh G, Bhalla A, Capalash N, Sharma P (2010) Characterization of immobilized laccase from γ-proteobacterium JB: approach towards the development of biosensor for the detection of phenolic compounds. Indian J Sci Technol 1:48–53
Singh G, Bhalla A, Kaur P, Capalash N, Sharma P (2011a) Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol 10:309–326
Singh G, Bhalla A, Ralhn PK (2011b) Extremophiles and extremozymes: importance in current biotechnology. ELBA Bioflux 3:46–54
Uzan E, Nousiainen P, Balland V, Sipila J, Piumi F, Navarro D, Asther M, Record E, Lomascolo A (2009) High redox potential laccases from the ligninolytic fungi Pycnoporus coccineus and Pycnoporus sanguineus suitable for white biotechnology: from gene cloning to enzyme characterization and applications. J Appl Microbiol 108:2199–2213
Viikari L, Ranua M, Kantelinen A, Sundquist J, Linko M (1986) Bleaching with enzymes. In: Proceedings of the 3rd International conference of biotechnology on pulp and paper industry, Stockholm, Sweden. 67–69
Virk AP, Sharma P, Capalash N (2012) Use of laccase in pulp and paper industry. Biotechnol Prog 28:21–32
Virk AP, Puri M, Gupta V, Capalash N, Sharma P (2013) Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS ONE 8(8):e72346. doi:10.1371/journal.pone.0072346
Vivekanand V, Dwivedi P, Sharma A, Sabharwal N, Singh RP (2008) Enhanced delignification of mixed wood pulp by Aspergillus fumigatus laccase mediator system. World J Microbiol Biotechnol 24:2799–2804
Weihua Q, Hongzhang C (2008) An alkali-stable enzyme with laccase activity from endophytic fungus and the enzymatic modification of alkali lignin. Bioresour Technol 99:5480–5484
Xu F, Shin W, Brown SH, Wahleitner JA, Sundaram UM, Solomon EI (1996) A study of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Yaropolov AI, Skorobogatko OV, Vartanov SS, Varfolomeyev SD (1994) Laccase: properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol 49:257–280
Zhang C, Zhang S, Diao H, Zhao H, Zhu X, Lu F, Lu Z (2013) Purification and characterization of a temperature- and pH-stable laccase from the spores of Bacillus vallismortis fmb-103 and its application in the degradation of malachite green. J Agric Food Chem 61:5468–5473
Zheng Z, Li H, Li L, Shao W (2012) Biobleaching of wheat straw pulp with recombinant laccase from the hyperthermophilic Thermus thermophilus. Biotechnol Lett 34:541–547
Acknowledgments
Authors are thankful to SERB/DST, Delhi, India, for providing the research funding under fast track young scientist program (SB/FT/LS-315/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, G., Kaur, K., Puri, S. et al. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol 99, 155–164 (2015). https://doi.org/10.1007/s00253-014-6219-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6219-0