Abstract
Recombinant Escherichia coli cells were applied for the recovery of electric energy from formate. Initially, the fdh gene, which encodes formate dehydrogenase (FDH) of Mycobacterium vaccae, was introduced into E. coli cells to allow efficient degradation of formate. The constructed microbial fuel cell (MFC) with E. coli BW25113 cells carrying fdh gene showed appreciable generation of current density in the presence of formate as a substrate. Current density and polarization curves revealed that the performance of MFC under examined conditions was limited by the electron transfer from bulk liquid to the electrode surface; accordingly, agitation resulted in an increase in the current density and achieved a coulombic efficiency of 21.7 % on the basis of formate consumed. Thus, gene recombination enables E. coli cells to utilize formate as a fuel for MFC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of energy from renewable substrates is important for achieving sustainable energy supply. Microbial fuel cells (MFCs) are devices that convert the chemical energy of biological fuels into electric energy using microorganisms as biocatalysts [1, 2]. MFCs have several key advantages over conventional fuel cells, including biofuel cells. For example, MFCs can be operated under mild conditions, and do not require expensive inorganic catalysts or purification of the biocatalysts [3]. Moreover, they allow large-scale and long-lifetime operation. When we discuss the industrial realization of MFCs, it is more realistic to operate MFCs in combination with wastewater treatment. According to an estimate, the energy used for wastewater treatment comprises approximately 1 % of the total energy consumption of Japan. Thus, recovering energy from organic compounds in wastewater would contribute to a more sustainable society.
Formic acid is one of the organic compounds included in wastewater. In Japan, it was reported that the estimated formate concentration in wastewater is ~80 mg l−1 [4]. There are several attempts to utilize formate as a fuel for MFCs using a mixed culture of Acetobacterium and Arcobacter spp. [5] or a pure culture of Geobacter sulfurreducens [6], one of microorganisms applied extensively in MFCs. Meanwhile, Escherichia coli has been also a promising candidate used in MFCs, where chemical mediators have been employed for transferring intercellular electrons to an electrode [7, 8]. Though E. coli has a broad spectrum in the utilization of organic compounds, this bacterium is generally unable to degrade formate because of the insufficient activity of inherent formate dehydrogenase (FDH). In a previous study, we achieved effective utilization of formate by recombinant E. coli cells with the introduction of fdh gene [9, 10]. Changing the redox status of E. coli cells accompanied with formate degradation enhanced the fermentative production of pyruvate and alanine. These results led us to a possible idea of generating electricity via formate degradation using recombinant E. coli cells.
In the present study, we examine the recovery of electric energy from formate by introducing the foreign fdh gene into E. coli cells. A MFC was conducted and operated to elucidate the potential of the recombinant E. coli cells for energy generation from formate.
Materials and methods
Bacterial strains
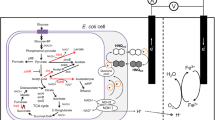
Escherichia coli BW25113 was obtained from the National BioResource Project (NIG, Mishima, Japan). The fdh gene from Mycobacterium vaccae was introduced into the E. coli BW25113 strain by using a pHSG399 plasmid as described previously [9], and the transformant was denoted as BW25113/pHfdh.
MFC operation
A two-chambered MFC was fabricated using two 300-ml Pyrex glass bottles with hand-made modifications. A 4.9-cm2 Nafion® perfluorinated membrane (Sigma–Aldrich, MO, USA) was clamped between the adapters. The cathode and anode were graphite unrolled by 0.20 g of untreated carbon cloth (TR3110MS, Mitsubishi Rayon Co., Tokyo, Japan). The effective surface areas of cathode and anode were 5.7 cm2 each. Copper wires were used to connect the circuit, and the connections were secured with alligator clips. The anode chamber was filled with 285 ml of M63 medium comprising of 2 g sodium formate, 1 g yeast extract, 2 g (NH4)2SO4, 13.6 g KH2PO4, 0.25 g MgSO4·7H2O, 0.5 mg FeSO4·7H2O, and 1 mg thiamine per liter of deionized water. It also contained 5 mmol l−1 2-hydroxyl-1,4-naphthoquinone (HNQ) as an electron transfer mediator [11]. The initial pH of the medium was 8.0. The cathodic chamber was filled with 285 ml of 100 mmol l−1 phosphate buffer (pH 8.0) containing 320 mmol l−1 potassium ferricyanide. The MFC was autoclaved at 120 °C for 20 min before use. E. coli cells were pre-cultured in lysogeny broth (LB) medium and harvested by centrifugation. The concentrated E. coli cell suspension was added to the anodic chamber at a concentration of 0.65 g-dry cells l−1. The MFC was operated at room temperature (ca. 25 °C) and the voltage was measured with a potentiostat (HA-303, Hokuto Denko Co., Tokyo, Japan) by connecting to a 200-Ω resistor. The anode chamber was agitated at 460 rpm with a magnetic stirrer when necessary. Polarization data were obtained by varying the external load with resistors ranging from 10 to 2,000 Ω.
The coulombic efficiency, E C, was calculated as E C = 100 × C P/C T [12], where C P is the total Coulombs calculated by integrating the current over time, and C T is the theoretical amount of Coulombs available from formate, which is given by the following equation.
where F is the Faraday’s constant (98,485 C per mole of electrons), N is the number of mole of electrons generated per mole of formate (2 electrons mol−1), ΔS is the concentration of formate consumed, v is the liquid volume, and M is the molecular mass of formate.
Analyses
The concentration of formate was measured by means of high-performance liquid chromatography (Shimadzu Corp. Kyoto, Japan) using an Aminex HPX-87H column (Bio-Rad Laboratories, Inc., Hercules, CA), in which a mobile phase of 20 mM H2SO4 was made to flow at 0.6 ml min−1 and 55 °C, and a UV detector working at 210 nm.
Results and discussion
Performance of the MFC and consideration of rate-limiting factor
The current density of the constructed MFC with or without E. coli BW25113/pHfdh cells is shown in Fig. 1. In the absence of E. coli cells (medium only), the value of current density was about 0.005 mA cm−2 even though the medium contained all components except E. coli cells, indicating that the background electricity of the constructed MFC is negligible. On the other hand, the appreciable current density was detected in the presence of BW25113/pHfdh cells. The current density gradually increased and reached about 0.08 mA cm−2 at 100 min, indicating that BW25113/pHfdh cells convert formate to electric energy. Regarding the reason for the gradual increase in current density, it can be considered that it takes time to establish a steady state of electron transfer from E. coli cells to the electrode under the examined conditions. Further increasing the formate concentration from 2 to 4 g l−1 did not result in a significant current increase, suggesting that the substrate supply does not limit the output of this system.
Next, we attempted to elucidate a rate-limiting step for increasing the output of the constructed MFC. For this purpose, polarization and power density curves were examined; this technique has been frequently used in previous studies to understand the performance of MFCs [13–15]. Figure 2 shows typical current and power density curves with varying external resistance in the presence of BW25113/pHfdh cells and formate. As a result, a significant increase in current was not observed with changing voltage and the value remained constant at around 0.1 mA cm−2, which clearly indicates that the electron transfer from bulk liquid to the electrode surface in the anode chamber is limited under the experimental conditions. To diminish the limitation of diffusion, the anode chamber was agitated with a magnetic stirrer. The rate of agitation was fixed at 460 rpm to ensure well mixing in the chamber. With agitation, the current density increased with decreasing voltage and reached about 0.53 mA cm−2 near the open circuit. These results show that agitation alleviates the rate-limiting step of electron transfer. From the power density curves, the maximum power density was determined to be 0.076 mW cm−2.
Improvement of MFC performance
Considering the results displayed in Fig. 2, the extended time profiles of current density was then examined under the agitating condition in the anode chamber (Fig. 3). As expected, the initial value of current density drastically increased to about 0.3 mA cm−2 and the stable output was confirmed throughout the examined period. In the case of parent BW25113 cells as a negative control, the current density transiently increased to 0.05 mA cm−2 and then decreased to 0.001 mA cm−2 at 3 h with no appreciable increase afterward. For the transient increase of current density at the initial phase, it is thought that intracellular electrons carried over from pre-cultures were released from the cells via HNQ.
Table 1 summarizes the performance of the constructed MFC with BW25113 or BW25113/pHfdh cells under agitating conditions. In the case of BW25113 cells, formate consumption was not detected for 18 h, which suggests that the inherent FDH of the wild-type strain could not degrade formate under the examined conditions. In contrast, in the case of BW25113/pHfdh cells, the consumption of formate was 0.45 ± 0.10 g l−1 for 18 h. Mean current density was 0.318 mA cm−2 and the E C value achieved 21.7 %. To our knowledge, this is the first report of energy production from formate with the engineered E. coli cells.
Conclusion
E. coli BW25113/pHfdh cells were applied for MFC to generate electric energy from formate as a substrate. The polarization curves revealed that the reaction rate under the experimental conditions was limited by the electron transfer from bulk liquid to the electrode surface in the anode chamber. Agitation eliminated the diffusion-derived limitation and enabled a coulombic efficiency of 21.7 % with formate. Thus, gene recombination allows E. coli cells to utilize formate as a fuel for MFCs.
References
Kim BH, Chang IS, Gadd GM (2007) Challenges in microbial fuel development and operation. Appl Environ Microbiol 76:485–494
Pant D, Bogaert GV, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9:2619–2629
Matsui S, Ide S, Otaki Y (1983) Roles of sulfate-reducing bacteria in organic matter decomposition and acetic acid formation by the anaerobic fluidized bed treatment. Proc Environ Sani Eng Res 19:156–162
Ha PT, Tae B, Chang IS (2008) Performance and bacterial consortium of microbial fuel cell fed with formate. Energy Fuel 22:164–168
Speers AM, Reguera G (2011) Electron donors supporting growth and electroactivity of Geobacter sulfurreducens anode biofilms. Appl Environ Microbiol 78:437–444
Potter MC (1912) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc Lond Ser B 84:260–276
Liu J, Yong YC, Song H, Li CM (2012) Activation enhancement of citric acid cycle to promote bioelectrocatalytic activity of arcA knockout Escherichia coli toward high-performance microbial fuel cell. ACS Catal 2:1749–1752
Ojima Y, Suryadarma P, Tsuchida K, Taya M (2012) Accumulation of pyruvate by changing the redox status in Escherichia coli. Biotechnol Lett 34:889–893
Suryadarma P, Ojima Y, Tsuchida K, Taya M (2012) Design of Escherichia coli cell culture for regulating alanine production under aerobic conditions. J Chem Eng Jpn 45:604–608
Wakisaka T, Takada Y, Azuma M (2009) Power generation characteristics and performance improvement of a bio-fuel cell using yeast. Kouongakukaishi 35:283–290 (in Japanese)
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Qiao Y, Bao SJ, Li CM, Cui XQ, Lu ZS, Guo J (2008) Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano 2:113–119
Sakai H, Nakagawa T, Tokita Y, Hatazawa T, Ikeda T, Tsujimura S, Kano K (2009) A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ Sci 2:133–138
Nimje VR, Chen C-Y, Chen C-C, Chen H-R, Tseng M-J, Jean J-S, Chang Y-S (2011) Glycerol degradation in single-chamber microbial fuel cells. Bioresour Technol 102:2629–2634
Acknowledgments
This study was supported in part by Kansai Research Foundation for Technology Promotion. This study was also founded by the Sumitomo Foundation. We thank Dr. Kurihara of Kyoto University for kindly providing the pFDHADH plasmid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ojima, Y., Kawata, T., Matsuo, N. et al. Recovery of electric energy from formate by using a recombinant strain of Escherichia coli . Bioprocess Biosyst Eng 37, 2005–2008 (2014). https://doi.org/10.1007/s00449-014-1179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1179-4