Abstract
A high titre of thermo-alkali-stable xylanase was attained in cane molasses medium. When the culture variables for endoxylanase production were optimized [cane molasses 7 %, soluble alkaline extract of wheat bran (SAE-WB) 37 % and ammonium chloride 0.30 %], a 4.5-fold enhancement in xylanase production (69 U ml−1) was achieved as compared to that in the unoptimized medium (15 U ml−1). The enzyme titre attained in shake flasks could be sustained in a 7-l laboratory bioreactor. An activity band corresponding to 40 kDa was visualized on SDS-PAGE zymogram analysis. The enzyme has broad range of pH and temperature for activity with optima at 9.0 and 80 °C, and stable between pH 4.0 and 11.0 with 85 % retention of activity. It has T 1/2 of 40 and 15 min at 70 and 80 °C. The enzyme is halotolerant since it displays activity in the presence of salt up to 15 %, and remains 100 % active in the absence of salt. The supplementation of whole wheat dough with xylanase improves antistaling property, reducing sugar content, bread volume with prebiotic xylooligosaccharides in bread. This is the first report on xylanase production in cane molasses medium with SAE-WB as the inducer and its applicability in whole wheat bread making that improves human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hemicelluloses are amorphous branched heteropolymers which are mainly made of pentoses. These heteropolymers represent about 20–40 % of plant biomass, and xylan is the major polymer having the backbone of β-1,4-linked d-xylose residues. The complete degradation of xylan requires the synergistic action of several enzymes [1]. Endo-1,4-β-xylanases (EC 3.2.1.8) cleave the xylan backbone, and the resulting xylooligomers are further hydrolyzed into xylose by exo-acting β-xylosidases (EC 3.2.1.37). Xylanases find applications in the production of bioethanol, aroma, fruit juices, animal feeds, baking, textiles, leather, and recycling of waste paper besides their utility in pre-bleaching of paper pulps [2].
The enzymes have been used in biotechnological processes since early days. Enzymes are now used in bread making and applications of enzymes in this field are expected to rise at a much faster rate in the future. Several commercial enzyme preparations are currently in use in baking industry, and xylanases find widespread utility in bread making [3]. In the modern society, consumers’ expectations as well as food-related needs have changed substantially. In order to have more fibre-rich wholesome foods, the baking industry is shifting towards the production of whole wheat bread with minimum or without chemicals. There are, however, certain technological difficulties related to the production of such breads. The development of appropriate technology is required for making whole wheat bread with improved body texture, flavour and other desirable properties which will improve the consumers’ acceptance. Microbial enzymes can be of great help in developing such technologies by including these as process aids/additives [4].
In flour, generally arabinoxylan content varies from 2 to 5.0 % in whole wheat meal along with 80 % starch and 12 % proteins. The arabinoxylan, although present in minor amounts, is an extremely important functional ingredient as it can bind almost ten times its own weight of water, and hence, exerts a significant effect on the flour and dough, and bread quality [5, 6]. The extremely strong water holding capacity of arabinoxylans in wheat flour has a negative effect on dough quality, being detrimental to the formation of the protein films in the dough due to physical interruption [7, 8]. The solubilisation of insoluble arabinoxylan to give high molecular weight solubilised xylooligosaccharides would lead to a net loss of water holding capacity [9]. This hydrolysis not only removes the insoluble arabinoxylan that interferes with the formation of the gluten network in dough, but also enhances the stability of the dough system by increasing the viscosity. This in turn yields a more stable and flexible dough with a larger loaf volume, improved crumb structure (fine crumbs and thin gas cell walls), and hence, also a softer crumb [10]. Endo-1,4-xylanase (EC 3.2.1.8) randomly attacks the arabinoxylan backbone to cause a decrease in the degree of polymerization, and improves dough machinability, dough stability, oven spring, loaf volume, crumb structure and shelf life [4, 11]. As a consequence of the hydrolytic action of endoxylanases, xylooligosaccharides (XOs) are released, leading to in situ enrichment of prebiotic content, and thus, making bread healthier and fibre rich.
The economy of any biotechnological process depends on the cost and availability of suitable biocatalysts. Microorganisms are well known to produce high titres of xylanases [2]. The use of industrial byproducts in fermentation medium provides a cheaper alternative to energy source and further reduces the problems encountered with the management of industrial byproducts [12]. Molasses, a byproduct of sugar industry, contains high sugar concentration, vitamins and other micronutrients necessary for the microbial growth and fermentation process besides being less expensive. Molasses has been successfully used in various microbial fermentation processes [13–15].
The biosynthesis of hemicellulases is inducible in nature [16, 17]. The use of commercial inducers like lactose, cellobiose, xylose and xylan is not economical for large-scale production of industrial enzymes. Furthermore, the use of agro-residues creates a technical problem in fermentation [18]. In this respect, the use of soluble alkali extract of lignocellulosic materials from agricultural residues has been recommended for developing industrial enzyme production technologies [16]. The optimization of cultural variables can further improve the efficiency of fermentation. The classical ‘one-variable-at-a-time’ approach is an effective technique of optimization, but fails to depict the combined or interactive effect of the variables. The statistical optimization using response surface methodology (RSM) has, therefore, become an important tool for process optimization and/or media formulation. The use of this software-based technique makes the process faster and allows understanding the interactions between variables and their effects on the product yield [14, 15, 18].
The novel polyextremophilic bacterium Bacillus halodurans TSEV1 produces xylanase in an inducible manner, having stability in industrial process extremes and biotechnological applications [17–19]. Wheat flour is known to contain some proteinaceous xylanase inhibitors, and therefore, the use of additional xylanase from a polyextremophilic microbe in bread will be beneficial. The production of xylanase in B. halodurans TSEV1 using wheat bran as the sole carbon source was attempted, but the enzyme titre was low in the laboratory fermenter [18]. This investigation is, therefore, focused on the optimization of xylanase production in cane molasses medium with soluble alkali extract of wheat bran (SAE-WB) as an economical inducer. The xylanase was partially purified, characterized and its applicability in whole wheat bread making was confirmed.
Materials and methods
Microorganism and other materials
The bacterial strain Bacillus halodurans TSEV1, used in this investigation, was isolated from an effluent sample collected from the Century Pulp and Paper Mill, Lal Kuan (Uttarakhand, India) and identified based on polyphasic approach (GenBank accession no. HQ184470) and deposited at Microbial Type Culture Collection Centre (MTCC), Institute of Microbial Technology, Chandigarh (MTCC 10962). The bacterium was routinely grown on Horikoshi modified agar medium at pH 10.5 and 50 °C and preserved as glycerol stocks at −20 °C [17].
All chemicals used were of AR grade and procured from Sigma (St. Louis, USA), Merck (Darmstadt, Germany), Qualigens, Hi-media (Mumbai, India), Promega (Medison, USA), Megazyme (Ireland), Geneaid (USA), while cane molasses and wheat bran were procured from the local market.
Enzyme assays
The enzyme was assayed by determining reducing sugars liberated by its action on birch wood xylan (Sigma) using dinitrosalicylic acid (DNSA) reagent. Xylanase was assayed according to Archana and Satyanarayana [20] at pH 9.0 and 80 °C. One unit of xylanase is defined as the amount of enzyme that liberates 1 μmol of reducing sugars as xylose min−1 under the assay conditions.
Optimization of enzyme production by one-variable-at–a-time approach
Cane molasses was incorporated into the production medium at 2.0–9.0 % (v/v), and 0.1 % xylan as inducer. Erlenmeyer flasks (250 ml) containing 50 ml medium (pH 9.0) were inoculated with 2 % of 16-h-old B. halodurans and incubated at 45 °C for 48 h. The bacterial growth was monitored by measuring absorbance at 600 nm. The cell-free supernatants after centrifugation at 10,000×g for 10 min were used in xylanase assays.
As the nitrogen level was less in the cane molasses medium (cane molasses 7 % v/v), it was supplemented with various organic (urea, tryptone, peptone, beef extract, yeast extract) and inorganic (ammonium sulphate, sodium nitrate, ammonium nitrate and ammonium chloride) nitrogen sources (0.09 g equivalent nitrogen per litre). The level of NH4Cl (0.1.0–0.5.0 %), the effect of initial pH (8.0–12.0) of medium (adjusted using 3 N HCl/NaOH), different surfactants and level of SAE-WB (prepared according to Sahai et al. [21]) as inducer (25.0–40.0 % v/v) were optimized in a similar manner.
Optimization of enzyme production in cane molasses medium using response surface methodology (RSM)
In order to improve enzyme production in cane molasses medium, central composite design (CCD) approach of RSM was used to determine the optimum levels and understanding the interactions between critical variables identified during single variable optimization as A. Cane molasses (% v/v), B. NH4Cl (% w/v), and C. SAE-WB (% v/v). A set of 20 experiments were performed with each independent variable in the design studied at five different levels (−α, −1, 0, +1, +α) (Table 1). Optimum values of each parameter obtained after one-variable optimization was considered as central value (0).
All 20 experiments were performed in triplicates and the average of xylanase titres attained was used as the dependent variable or response (Y). The xylanase production was analysed by using second order polynomial equation given below and the behaviour of the system was explained:
where, Y is predicted response, β 0 is intercept, β 1, β 2, β 3, and β 4 are linear coefficients. β 1,1, β 2,2, β 3,3, β 4,4 are squared coefficients. β 1,2, β 1,3, β 1,4, β 2,3, β 2,4, β 3, β 4 are interaction coefficients, and A, B, C, D, A 2, B 2, C 2, D 2, AB, AC, AD, BC, BD and CD are independent variables.
Statistical analysis of the model equation was determined by Fisher’s t value and the proportion of variance explained by the model was given by the multiple coefficient of determination, R squared (R 2) value. With the help of Design Expert Software version 6.0, three-dimensional curves were generated. The experiments were carried out according to CCD of RSM. Validation of the model was carried out in shake flasks under conditions predicted by the model.
Production of xylanase in laboratory fermenter
The xylanase was also produced in 7-L stirred tank laboratory fermentor (Applikon Biotechnology, The Netherlands) containing 4 L cane molasses-SAE-WB medium optimized by the statistical approach. The fermenter was operated at 45 °C, 200 rpm and pH 9.0 with 1 vvm aeration.
Purification and characterization of xylanase
The cell-free culture supernatant was passed through 10 kDa ultrafiltration membrane cartridge (Millipore), and the desalting and reconcentration were carried out for three cycles using the same membrane cartridge with 10 mM glycine–NaOH buffer (pH 9.0). This xylanase preparation was used for the characterization and application studies.
Zymogram analysis
The zymogram analysis was performed by loading the concentrated partially purified xylanase fraction on 12 % SDS-polyacrylamide gel containing 0.1 % xylan. After electrophoresis, the gel was washed for 30 min in double deionised water for removal of SDS. The washed gel was incubated for 60 min at 80 °C, soaked in 0.1 % Congo red solution for 30 min at room temperature and washed with 1 M NaCl till excess dye was removed. The activity band was seen as clear colourless area of xylan hydrolysis on the gel.
Effect of pH and temperature on xylanase activity and stability
Xylanase activity was determined using 1 % (w/v) birchwood xylan prepared in buffers of varied pH range 4.0–12.0 [0.1 M acetate buffer (pH 4.0–5.0), 0.1 M phosphate buffer (6.0–8.0), and glycine–NaOH (9.0–12.0)]. To assess the stability at different pH, the enzyme was incubated for 30 min in buffers of varied pH (4.0–12.0) and then the residual xylanase activity was determined under standard assay conditions.
The optimum temperature for the enzyme activity was determined by performing xylanase assays at different temperatures (30–100 °C, pH 9.0). The thermostability of xylanase was assessed by incubating the enzyme solution at 60, 70 and 80 °C, and the aliquots were drawn at regular time intervals for determining the residual enzyme activities.
Effect of sodium chloride on xylanase stability
To assess salt tolerance, xylanase was diluted to 20-fold with buffer (pH 9.0) having different concentrations of sodium chloride ranging from 0 to 20 %, incubated for 1 h at room temperature and the residual activities were determined.
Application of xylanase of B. halodurans in whole wheat bread making
Wheat flour (650 g) was mixed with dry yeast (4.5 g), NaCl (1.5 %, w/w) and 500 mg of xylanase of B. halodurans (50.0 U enzyme with specific enzyme activity of 0.10 U mg−1 protein) blended with 60 % (w/v) water and thoroughly mixed mechanically for 30 min to produce the dough, which was kept aside for proofing followed by fermentation for 1 h and baking at 275 °C for 30 min, followed by cutting and shaping. This process was performed with/without xylanase of B. halodurans TSEV1. The breads were then assessed for reducing sugar, protein and moisture contents, softness and shelf life at the desired intervals.
Analysis of xylooligosaccharides in bread
The products liberated by the action of B. halodurans TSEV1 xylanase on wheat flour in the bread were analysed by thin layer chromatography (TLC). The control and test breads were suspended in deionized water (1 mg/ml) and the supernatants after centrifugation were analysed for sugars by spotting on silica gel 60 plates (Merck, Darmstadt, Germany), developed in n-butanol:ethanol:water (5:3:2 v/v), air dried and sprayed with aniline-diphenylamine reagent, kept at 100 °C for 1 h and observed for the appearance of blue spots against white background.
All experiments were carried out in triplicate and the average values with standard deviation are presented.
Results and discussion
Members of the genus Bacillus and Geobacillus are known to produce a variety of extracellular enzymes, of which xylanases are of significant industrial importance [2, 22, 23]. In general, enzymes produced by thermophilic microbes are more thermostable than their mesophilic counterparts [24]. Cellulase-free xylanases have been reported from extremophilic bacterial strains such as Bacillus licheniformis A99 [20], G. thermoleovorans [23] and G. thermodenitrificans [25], but most of them are not adequately alkali stable. The cellulase-free xylanase produced by the bacterial strain B. halodurans TSEV1 has both alkali stability and thermostability [19] along with broad pH and temperature ranges for activity, suggesting its potential application in biotechnological processes operated under extreme conditions.
Although solid-state fermentation (SSF) is considered to have advantages such as the reduction of contamination due to heavy inoculum used, lower capital investment and easy downstream processing over submerged fermentation (SmF), 90 % of the industrial enzymes are produced by SmF [26]. The production of high titres of enzymes by optimizing the process variables is of prime importance in industrial enzymology. The optimization of various nutritional and physical parameters is well known to increase production levels significantly. Carbon sources in the medium play profound role in the enzyme production behaviour of the microbes. There is no general defined medium for xylanase production by different microbial strains. Every microorganism has its own physico-chemical and nutritional requirements. In view of the commercial utility of the xylanase, formulation of a cost-effective medium is necessary. Molasses is one of the cheapest sources of carbohydrates. Cane molasses (7 %) supported high xylanase titre as well as growth of the bacterium (Fig. S1). Molasses, besides a large amount of sugar (50 %), also contains nitrogenous substances (0.4–1.5 %), vitamins such as thiamine (830/100 g dry weight), pyridoxine (650/100 g), folic acid (3.8/100 g), biotin (120/100 g), pantothenic acid (2,140/100 g), amino nitrogen and trace elements (CaO 0.1–1.1 %; MgO 0.03–0.1 %; K2O 2.6–5.0 %) [27]. Cane molasses (7 %) supported a high xylanase titre as reported for α-amylase production by Geobacillus thermoleovorans [14]. It could be attributed to the presence of micronutrients and vitamins in molasses which are generally required for the growth of extremophiles.
The pH of the medium is well known to affect many enzymatic processes and transport of various components across the cell membrane. Most of the known microbial xylanases are optimally produced at pH 5.5–9.5 [28]. A high titre of xylanase was produced by B. halodurans TSEV1 at pH 10.0 (Fig. S2) as reported in other bacterial strains [23, 29]. The production of xylanase by B. halodurans TSEV1 was pH dependent. Interestingly, irrespective of initial pH, the final pH of the medium was shifted to 9–9.5 that appears to be an adaptation for alkaline pH [30]. Xylanase synthesis in B. halodurans TSEV1 is inducible [17], and therefore, medium must be supplemented with xylan, which is costly. Soluble alkali extract of wheat bran at 35 % (Fig. S3) induced higher enzyme titre in B. halodurans, similar to that reported in Melanocarpus albomyces IITD3A (Biswas et al. [16]). The pH adjustments made by the addition of acid/alkali to the medium incurs cost. By supplementing the medium with SAE-WB, the initial pH of medium becomes 10.0, which further makes the process economical due to elimination of pH adjustment step. The optimum levels of other parameters that significantly affect xylanase production and growth of bacterium include NH4Cl (0.3 %), Tween-40 (0.1 %) and agitation (250 rpm) (Figs. S4–S8). Supplementation of cane molasses with 0.3 % NH4Cl supported higher xylanase titre (Fig. S5). Similarly, 0.22 % urea supported highest α-amylase production in G. thermoleovorans [14]. Surfactants are known to affect the growth and enzyme production in microbes [31], and therefore, have been used in biotechnology for improving the yield of a number of enzymes produced by fermentation. Among surfactants, a high xylanase titre was attained in the medium containing Tween-40 (0.1 %) (Fig. S6). At higher concentrations, the enzyme production declined, which could be due to increased viscosity of the medium that results in decreased oxygen transfer rate [15]. Based on the observations of ‘one-variable-at-a-time’ approach, the concentrations of three significant variables (cane molasses, ammonium chloride and SAE-WB) were optimized using CCD of RSM.
The results of CCD experiment used for studying the effect of three independent variables are given with mean predicted and observed responses (Table 2). The regression equations obtained after the analysis of variance (ANOVA) represents the level of xylanase production as a function of the initial values of level of cane molasses (A), ammonium chloride (B) and SAE-WB concentration (C). The response equation that suggests a suitable model for xylanase production is as follows:
The F value of 59.69 and ‘Prob > F’ value <0.0001 indicated that the model is significant. Coefficients A, B, C, A 2, B 2 and interaction of A with B and A with C are the significant model terms. The results of response surface model fitting in the form of analysis of variance (ANOVA) are given in Table S1. The coefficient of determination (R 2) is 0.9884 for xylanase production, which is close to the predicted R 2 (0.8946) and adjusted R 2 (0.9653). The adequate precision value of 30.03 indicated an adequate signal and suggested that the model can be used to navigate the design space. The 3-D response surface curves drawn for the interaction between SAE-WB and cane molasses, and SAE-WB and ammonium chloride suggested induction of xylanase by SAE-WB. A 4.6-fold enhancement in xylanase titers (69 U ml−1) attained due to statistical optimization is consistent with that reported for the production α-amylase by Geobacillus thermoleovorans [14] and phytase production in Sporotrichum thermophile in cane molasses medium [15]. To the best of our knowledge, this is the first report on xylanase production by any bacterium in cane molasses medium, which suggests technology development for economical production of xylanase.
Validation of the model and production in a laboratory fermenter
The experimental model and regression equation were validated by carrying out xylanase production in the optimized medium (Table 3). The enzyme production predicted by the quadratic model and that recorded experimentally are in good agreement, and thus, the variables optimized by statistical optimization can be used in large-scale xylanase production. The production of xylanase was sustained in shake flasks up to 1 L followed by a marginal decline and enhancement in the fermenter (Table 3). A peak in enzyme production was attained in 36 h in the fermenter as compared to that in flasks in 48 h (Fig. 1c). There are similar reports on enhanced/sustained enzyme production in the fermenter and reduction in fermentation time due to improved process conditions [14, 16]. The problem encountered with the particulate nature of wheat bran [18] could be overcome using cane molasses medium with SAE-WB. The enzyme productivity in the cane molasses was higher (1.9 U ml−1 h−1) than that in the wheat bran (1.15 U ml−1 h−1).
Purification and characterization of xylanase
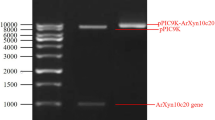
A high state of purity is generally required for analytical purposes and not for applications in industries like food processing, detergent as well as paper and pulp due to economical reasons. However, it is necessary to exclude certain other unwanted proteins [32]. The cyclic desalting and concentration of crude xylanase using 10 kDa membrane cartridge led to 72 % xylanase yield with reduced intensity of colour of cane molasses. The identity of xylanase was confirmed by SDS-PAGE-zymogram analysis where single activity band corresponding to 40 kDa protein band was observed (Fig. 2). A 40-kDa molecular mass of xylanase is comparable to the reports available on xylanases from B. halodurans [19, 33].
Effect of pH, temperature and salt concentrations on xylanase activity and stability
Enzyme activity is markedly influenced by pH because substrate binding and catalysis are often dependent on charge distribution on both substrate and particularly enzyme molecules [34]. The xylanase of B. halodurans TSEV1 has a broad range of pH (4.0–12.0) for activity with optimum at 9.0 (Fig. 3a). Remarkable enzyme stability was observed between pH 5.0 and 11.0 with about 85 % residual activity (Fig. 3b). The pH optimum of TSEV1 xylanase (pH 9.0) and remarkable enzyme stability observed is much better than or similar to the reports from other strains of B. halodurans [33, 35]. The broad range of pH activity and stability makes this enzyme a potent candidate for food and other biotechnological applications.
The optimum temperature for xylanase activity is 80 °C (Fig. 4a). The optimum temperature for xylanase activity is close to that of B. halodurans S7 [33] and G. thermoleovorans [23]. Utilization of enzymes in industrial processes often encounters thermal inactivation. The TSEV1 xylanase retained 100 % activity at 60 °C after 120 min, while at 70 °C, 40 % decrease in the activity was recorded after 30 min (Fig. 4b).The T 1/2 values of the enzyme at 70 and 80 °C are 40 and 15 min, respectively. The T 1/2 values of TSEV1 xylanase are better than those reported by Honda et al. [35] and Mamo et al. [33], for the xylanase of B. halodurans strains.
The high salt tolerance of this xylanase is of biotechnological interest. The xylanase is active over a broad range of NaCl concentrations (0–20 %) (Fig. 5). The salt tolerance of this enzyme is less than that of B. halodurans TSPV1 [36], but it retains 100 % activity in the absence of NaCl, suggesting the enzyme to be halotolerant [37]. An extremely halotolerant xylanase of Gracilibacillus sp. TSCPVG had been shown to be highly stable at variable salinities of 0–30 % NaCl [38]. Hung et al. [39] reported a salt-tolerant xylanase from Thermoanaerobacterium saccharolyticum NTOU1 that exhibited optimum activity at 0.4 M NaCl and tolerated up to 2 M NaCl. Very few reports are available on xylanases which tolerate three extremes simultaneously [36].
Application of xylanase of B. halodurans in whole wheat bread making
The final loaf volume is most important measure of bread quality. In case of whole wheat bread, loaf volume tends to be lower than the bread made from white flour, this is due to the less hydration of gluten in the presence of insoluble arabinoxylans [40]. The bread made by supplementing the dough with endoxylanase of B. halodurans (test) was soft with comparable moisture content, higher reducing sugars and soluble protein than the bread made without enzyme mix and emulsifiers (control) (Table 4). By the application of xylanase from B. halodurans, the dough rise was 1 cm more than that of control that results in increased specific volume as reported earlier [41, 42]. The improvement in bread volume could be attributed to hydrolysis of insoluble arabinoxylans and increment in gluten volume, which provides extensibility and results in better oven spring and increased loaf volume [42]. The crumb structure was better with the supplementation of enzyme than the control (Fig 6a). The shelf life of the test bread improved to 4 days at room temperature as compared to 2.5 days of the control bread because xylanases are also known to provide antistaling effect to the bread [6, 9]. Xylooligosaccharides have been detected in the test bread (Fig. 6b) that supports the hydrolysis of pentosans present in wheat flour by the action of xylanase. The hydrolysis of non-starch polysaccharides by xylanase will result in high digestibility and chewability of bread, and xylooligosaccharides as the hydrolysis product. The presence of XOs in bread would help in improving human health.
a Control bread made without xylanase, test bread made with xylanase of B. halodurans, a rise in crumb size, b improved texture of breads. b TLC analysis of the sugars released from test and control bread, 1 standards; xylose, xylotriose, xylotetraose, xylopentaose; 2 wheat flour dough with xylanase prior to baking; 3 control bread; 4 test bread
Conclusions
A significant enhancement in the production of thermo-alkali-stable endoxylanase by B. halodurans was achieved in cane molasses-SAE-WB medium as compared to that in the medium containing wheat bran. As cane molasses is a byproduct of sugar industries and SAE-WB is cheaper inducer than xylan, the cost of xylanase production would be lower than that of other xylanases. The supplementation of dough with TSEV1 xylanase improved bread quality, which is possibly due to the broad range of pH for activity as well as stability of the enzyme. The TSEV1 xylanase has also retarded the bread staling due to reduction in the initial crumb firmness and the firming process during storage. Being stable in extreme conditions and cellulase-free, TSEV1 xylanase can also find applications in other biotechnological processes.
References
Collin T, Gerday C, Feller G (2005) Xylanase, xylanase families and extremophilic xylanase. FEMS Microbiol Rev 29:3–23
Satyanarayana T, Sharma A, Mehta D, Puri AK, Kumar V, Nisha M, Joshi S (2012) In: Satyanarayana T, Johri BN, Anil Prakash (eds) Microorganisms in sustainable agriculture and biotechnology. Springer, Netherlands
Butt MS, Nadeem MT, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Hammer RJ (1995) In: Tucker G, Wood LFJ (eds) Enzymes in food processing, 2nd edn. Blackie Academic and Professional, Glasgow
Baillet E, Downey G, Tuohy M (2003) In: Courtin CM, Veraverbeke WS, Delcour JA (eds) Proceedings of the 3rd European Symposium on Enzymes in Grain Processing (ESEGP-3). Katholieke Universiteit Leuven, Leuven
Courtin CW, Delcour JA (2002) Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci 35:225–243
Gruppen H, Hamer RJ, Voragen AGJ (1992) Water-unextractable cell wall material from wheat flour. 2. Fractionation of alkali-extracted polymers and comparison with water-extractable arabinoxylans. J Cereal Sci 16:53–67
Wang M, van Vliet T, Hamer RJ (2004) Evidence that pentosans/xylanase affects the re-agglomeration of the gluten network. J Cereal Sci 39:341–349
Sorensen JF, Kragh KM, Sibbesen O, Delcour J, Goesaert H, Svensson B, Tahir TA, Brufau J, Perez-Vendrell AM, Bellincampi D, D’Ovidio R, Camardella L, Giovane A, Bonnin E, Juge N (2004) Potential role of glycosidase inhibitors in industrial biotechnological applications. Biochim Biophys Acta 1696:275–287
Qi Si J, Drost-Lustenberger C (2002) In: Whitehurst RJ, Law BA (eds) Enzymes in food technology. Sheffield Academic Press Ltd., Sheffield
Poutanen K (1997) Enzymes: an important tool in the improvement of the quality of cereal foods. Trends Food Sci Technol 8:300–306
Jin P, Bhattacharya SK, Williama CJ, Zhang H (1998) Effects of sulfide addition on copper inhibition in methanogenic systems. Water Res 32:977–988
Banik RM, Santhiagu A, Upadhyay SN (2007) Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol 98:792–797
Rao UJLM, Satyanarayana T (2007) Improving production of hyperthermostable and high maltose-forming a-amylase by an extreme thermophile Geobacillus thermoleovorans using response surface methodology and its applications. Bioresour Technol 98:345–352
Singh B, Satyanarayana T (2008) Phytase production by Sporotrichum thermophile in a cost-effective cane molasses medium in submerged fermentation and its application in bread. J Appl Microbiol 105:1858–1865
Biswas R, Sahai V, Mishra S, Bisaria VS (2010) Bioprocess strategies for enhanced production of xylanase by Melanocarpus albomyces IITD3A on agro-residual extract. J Biosci Bioeng 110:702–708
Kumar V, Satyanarayana T (2011) Applicability of thermo-alkali-stable and cellulase-free xylanase from a novel thermo-halo-alkaliphilic Bacillus halodurans in producing xylooligosaccharides. Biotechnol Lett 33:2279–2285
Kumar V, Satyanarayana T (2012) Thermo-alkali-stable xylanase of a novel polyextremophilic Bacillus halodurans TSEV1 and its application in biobleaching. Intr Biodet Biodegrad 75:138–145
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characterization of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17:797–808
Archana A, Satyanarayana T (1997) Xylanase production by thermophilic Bacillus licheniformis A99 in solid state fermentation. Enzyme Microb Technol 21:12–17
Sahai V, Mishra S, Bisaria VS (2005) Cellulose-free nutrient medium for enhanced productivity and activity of cellulase-free enzymes. Indian Patent 1246/DEL/2005
Khasin A, Alchanati I, Shoham Y (1993) Purification and characterization of a thermostable xylanase from Bacillus stearothermophilus T-6. Appl Environ Microbiol 59:1725–1730
Sharma A, Adhikari S, Satyanarayana T (2007) Alkali-thermostable and cellulase free xylanase production by an extreme thermophile Geobacillus thermoleovorans. World J Microbiol Biotechnol 23:483–490
Techapun C, Poosaran N, Watanabe M, Sasaki K (2003) Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochem 38:1327–1340
Anand A, Kumar V, Satyanarayana T (2013) Characteristics of thermostable endoxylanase and β-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles 17:357–366
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Crueger W, Crueger A (2000) In: Crueger W, Crueger A (eds) Biotechnology, a textbook of industrial microbiology. Panima Publisher Corporation, New Delhi
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Ko CH, Lin ZP, Tu J, Tsai CH, Liu CC, Chen HT, Wng TP (2010) Xylanase production by Paenibacillus campinasensis BL11 and its pretreatment of hardwood kraft pulp bleaching. Intr Biodet Biodegrad 64:13–19
Horikoshi K (1991) In: Horikoshi K (ed) Microorganisms in alkaline environments. Kodansha Limited, Tokyo
Nampoothiri KM, Tomes GJ, Roopesh K, Szakacs G, Nagy V, Soccol CR, Pandey A (2004) Thermostable phytase production by Thermoascus aurantiacus in submerged fermentation. Appl Biochem Biotechnol 118:205–214
Price NC, Stevens L (1999) Fundamentals of enzymology: the cell and molecular biology of catalytic proteins. Oxford University Press, Oxford
Mamo G, Hatti-Kaul R, Mattiasson B (2006) A thermostable alkaline active endob-1-4-xylanase from Bacillus halodurans S7: purification and characterization. Enzyme Microb Technol 39:1492–1498
Shah AR, Madamwar D (2005) Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem 40:1763–1771
Honda H, Kudo T, Ikura Y, Horikoshi K (1985) Two types of xylanases of alkalophilic Bacillus sp. No. C-125. Can J Microbiol 31:538–542
Kumar V, Poonam S, Satyanarayana T (2013) Highly thermo-halo-alkali-stable β-1,4-endoxylanase from a novel polyextremophilic strain of Bacillus halodurans. Bioprocess Biosyst Eng 36:555–565
Wejse PL, Ingvorsen K, Mortensen KK (2003) Purification and characterization of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 7:423–443
Giridhar PV, Chandra TS (2010) Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillus sp. TSCPVG. Process Biochem 45:1730–1737
Hung KS, Liu SM, Fang TY, Tzou WS, Lin FP, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Biotechnol Lett 33:1441–1447
Lai CS, Davis AB, Hoseney RC (1989) Functional effect of bran in bread-making. J Cereal Chem 66:217–219
Maat J, Roza M, Verbakel J, Stem H, Santos da Silva MJ, Bosse M, Egmond MR, Hagemans MLD, Gorcom RFM, Hessing JGM et al (1992) In: Visser J, Beldman G, Austers-vanSomeran MA, Voragen AGJ (eds) Xylan and xylanases. Elsevier, Amsterdam
Jiang Z, Li X, Yang S, Li L, Tan S (2005) Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritima. Food Res Int 38:37–43
Acknowledgments
The authors thank Mr. Vijay Kumar Gupta (Tushar Nutritive Food Industry, New Delhi) for extending help in assessing the applicability of endoxylanase in whole wheat bread making. VK is grateful to Indian Council of Medical Research, Govt. of India, New Delhi, for awarding senior research fellowship while carrying out this investigation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Satyanarayana, T. Production of thermo-alkali-stable xylanase by a novel polyextremophilic Bacillus halodurans TSEV1 in cane molasses medium and its applicability in making whole wheat bread. Bioprocess Biosyst Eng 37, 1043–1053 (2014). https://doi.org/10.1007/s00449-013-1075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1075-3