Abstract
A xylanase gene was PCR-cloned from Thermoanaerobacterium saccharolyticum and expressed in Escherichia coli. The xylanase (XynA) consisted of a signal peptide, glycoside hydrolase family 10 domains, carbohydrate-binding modules, and surface layer homology domains. It was optimally active at 70–73°C and at pH 5–7. It had enhanced activity with NaCl with optimal activity at 0.4 M but was tolerant up to 2 M NaCl. The thermostable and salt-tolerant properties of this xylanase suggest that it may be useful for industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xylanases (EC 3.2.1.8) are a type of hemicellulase. They hydrolyze xylan, which are the most common hemicellulose in plants. In paper manufacturing, xylanases used in biobleaching have already displayed their potential as an environmentally friendly bleaching agent (Ziaie-Shirkolaee et al. 2008). The enzymes are also added to animal feed to increase animal’s digestive ability (Fan et al. 2009).
A number of xylanase genes have been isolated from mesophiles and the enzymes are characterized by optimal activities at 15–40°C and neutral pHs. Because extremophilic enzymes have been demonstrated to possess utility in many biotechnological processes, many xylanases have been obtained from thermophilic (50–80°C) to hyperthermophilic (>80°C) environments (van den Burg 2003). These enzymes have been applied in biotechnological processes in which high temperatures are required to process substrates. There is also a growing interest in salt-tolerant microbes living in environments such as salt pans (Oren 2002). However, salt-tolerance of xylanases has been rarely reported. Salt-tolerant xylanases derived from bacteria living in marine or saline environments have potential uses in industrial applications (Setati 2010), such as in the processing of sea food and food with a high salt content (Guo et al. 2009). In baking, salt-tolerant xylanases combined with salt have potentials in increasing the strength of the dough and adding flavor to baked goods (Butt et al. 2008).
Here, we report the expression and characterization of a thermostable endoxylanase from Thermoanaerobacterium saccharolyticum NTOU1, isolated from an acidic, hydrothermal vent near Gueishan Island, Taiwan. It is a Gram-negative bacterium, showing thermophilic, halophilic and anaerobic growth (Lin et al. 2010). The strain utilizes xylan as a carbon source in the presence of 0–4.5% (w/v) NaCl with an optimum pH of approx. 6.5. Functional genes of T. saccharolyticum NTOU1 were sequenced from genomic DNA and the gene of the glycosidase family 10 xylanase, termed XynA, was identified by genomic analysis. This gene was cloned and functionally expressed in E. coli. The recombinant XynA demonstrated thermostability and salt-tolerant characteristics.
Materials and methods
Construction of the expression vector for XynA
Thermoanaerobacterium saccharolyticum NTOU1 has been submitted to the Bioresource Collection and Research Center in Taiwan (strain number: BCRC 910455). The XynA gene was amplified by PCR with forward primer 5′-GTG GGA TCC ATG AAA AAT AAT GTA GAT AGG-3′ and reverse primer 5′-GCG TAC GCC GTC GAC TAG ATT ATT TGA TTT-3′, which contained restriction sites (underlined) for BamHI and SalI. Two primers were designed based on the XynA gene sequence of T. saccharolyticum NTOU1 (GenBank accession no. GQ868303). The PCR product was ligated into a pGEM-T vector (Promega) and transformed into E. coli DH5α. After the sequence of the entire XynA gene was confirmed, the XynA gene was digested with BamHI and SalI and ligated into the pET-23a(+) expression vector (Novagen), resulting in a recombinant vector designated as pET-23a-XynA.
Expression of the recombinant XynA
The pET-23a-XynA vector was transformed into E. coli BL21(DE3) and the clones were selected by plate activity assay using a Remazol Brilliant Blue (RBB)/xylan plate. The positive clones were isolated for XynA expression. The transformants were cultured overnight at 37°C in 5 ml Luria broth (LB) containing 100 μg ampicillin/ml. The cells were then added to 1 l LB medium and incubated at 37°C until OD600 reached 0.6 and the recombinant XynA was induced in the presence of 1 mM IPTG. After 6 h the cells were harvested by centrifugation at 4,000×g and suspended in 25 ml 20 mM phosphate buffer (pH 6.0). The cells were disrupted by ultra sonication. The lysate was heated at 65°C for 20 min and centrifuged at 12,000×g for 30 min to remove the heat-labile proteins. The supernatant was loaded onto a Ni-Sepharose 6 Fast Flow affinity column equilibrated with 10 mM phosphate buffer (pH 6.0) and the proteins were eluted with 4 volumes of the same buffer containing 250 mM imidazole. The fractions displaying xylanase activity were pooled and applied to a Sephacryl S-300 column. The fractions showing the enzymatic activity were collected and then loaded onto a Q Fast Flow column with phosphate buffer (pH 6.0). The bound proteins were eluted with a linear gradient of (NH4)2SO4 (0 to 100 mM) in a phosphate buffer (pH 6.0).
Proteins were separated by 10% SDS-PAGE with the gel being stained with Coomassie Brilliant Blue R-250. Xylanase activity in gel was performed by the zymogram technique. First, samples were resolved using 10% SDS-acrylamide gel containing 0.5% (w/v) soluble oat spelt xylan. After electrophoresis, the gel was washed twice with 50 mM sodium acetate buffer (pH 5.5) containing 25% (v/v) 2-isopropanol for 30 min and then soaked in a 50 mM sodium acetate buffer for 15 min. Then the gel was incubated in 50 mM sodium acetate buffer for 30 min at 60°C and stained in a 0.2% (w/v) Congo Red for 10 min at room temperature. Congo Red was destained with 1 M NaCl. Xylanase activity was detected as a light yellow band against a red background.
Xylanase activity assay
The xylanase activity was analyzed using the 3,5-dinitrosalicylic acid (DNS) method. The enzyme (2 μg) was added to 1 ml 4.5% (w/v) soluble beech wood, birch wood, and oat spelt xylan (Sigma) in 20 mM phosphate buffer at pH 5.5 and 72°C for 5 min. DNS reagent was then added to stop the reaction and the mixture was incubated at 100°C for 5 min. The absorbance at 540 nm was measured to determine the amount of reducing sugar with xylose as standards. One unit of xylanase activity was defined as the amount of enzyme required to produce 1 μmol reducing sugar in 1 min.
TLC was used to detect the hydrolysis of xylooligosaccharides. The reaction mixture consisted of 10 mM substrate (xylose, xylobiose, xylotriose, and xylopentose, respectively) and 1 μg of purified enzyme in 20 mM phosphate buffer (pH 5.5). The reaction was incubated at 70°C for 2 h then analyzed on a Merck Silica Gel 60F plate.
Effects of pH and temperature
The optimal pH for XynA was determined using buffers at 50 mM: citrate/NaOH buffer (pH 4.0–5.0), sodium phosphate buffer (pH 5.5–7.0), and Tris/HCl buffer (pH 7.5–8.0). The pH stability of XynA was determined after 24 h in the various buffers at 25°C in the absence of substrate. After incubation, xylanase activity was measured at 72°C and pH 5.5 by using beech wood xylan as a substrate. The optimal temperature was measured over a temperature range of 20–90°C at pH 5.5. To detect thermal stability, the recombinant xylanase was incubated in 20 mM sodium phosphate buffer (pH 5.5) for up to 120 min from 65 to 80°C in the absence of substrate. After incubation, the residual enzyme activity was determined at 72°C by using beech wood xylan as a substrate.
Effect of salt
The enzyme was also assayed with NaCl up to 0.5 M. Salt-tolerant activity was determined by incubating the purified XynA in a sodium phosphate buffer for 24 h at room temperature with NaCl up to 2 M. After incubation, the residual enzyme activity was determined at 72°C and pH 5.5 by using beech wood xylan as a substrate.
Results and discussion
Sequence analysis of XynA from T. saccharolyticum
Thermoanaerobacterium saccharolyticum has adapted to live at high temperatures and in a high-salt environment. For this reason, the enzymes from thermophilic and halophilic microorganisms are of interest for their possible industrial application. The nucleotide sequence of the XynA gene is 4,287 bp long and encodes a polypeptide containing 1429 amino acids. The gene was submitted to GenBank with accession number ID: GQ868303. XynA displays several features as shown in Fig. 1. In the N-terminal, XynA contains a signal peptide typically harboring the peptidase processing site, Val-X-Ala, which is involved in protein secretion (Tuteja 2005). XynA has four carbohydrate-binding modules (CBM, family 4_9 and family 9). CBM is essential for carbohydrate binding and enhances the catalysis efficiency of the enzyme toward insoluble carbohydrate substrates by absorbing onto the surface of the insoluble substrate (Tunnicliffe et al. 2005). Moreover, the linking of CBMs with a catalytic domain of xylanases to insoluble substrates such as plant cell walls is stronger than it is with xylanases not containing CBM. The catalytic domain of XynA belongs to the endo-1,4-β-xylanases of glycoside hydrolase family 10 (GH10). Finally, the C-terminal end of XynA harbors three repeat sequences known as the surface layer homology (SLH) domain. SLH mediates enzyme anchorage on the bacterial surface layer (S-layer) (Mesnage et al. 2000).
Features of the XynA polypeptide. The number indicates amino acid position in XynA. Domains are described as follows: 1–33 signal peptide(SP); 40–188 and 197–342 carbohydrate-binding modules family 4_9 (CBM); 399–671 family 10 glycoside hydrolase (Glyco_10); 682–854 and 857–1043 carbohydrate-binding modules family 9; 1250–1290, 1309–1349, and 1373–1416 surface layer homology (SLH) domains
Comparisons of the amino acid sequences of several xylanases
Thermoanaerobacterium saccharolyticum XynA shares 25–85% identities with other GH10 xylanases (Table 1) and 85 and 81% identities, respectively, with the xylanases from Thermoanaerobacterium saccharolyticum B6A-RI (native protein from Lee et al. 1993b; recombinant protein from Lee et al. 1993a) and Thermoanaerobacterium sp. JW/SL-YS 485 (native protein from Shao et al. 1995; recombinant protein from Liu et al. 1996). These two strains were isolated from the hot springs of Yellowstone National Park, whereas T. saccharolyticum NTOU1 was isolated from a hydrothermal vent in the ocean.
Cloning, expression, and purification of XynA
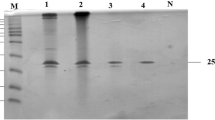
The XynA gene was cloned into the pET-23a(+) expression vector and expressed in E. coli BL21(DE3). The yield of active XynA expressed in E. coli was 1,34,000 U/l and 19,200 U/g of wet cells. The XynA was purified from the crude extract to homogeneity by successive Ni-affinity chromatography, Sephacryl S-300 chromatography, and Q Fast flow chromatography (Fig. 2a). In addition, the xylanase activity was identified by zymogram analysis (Fig. 2b). The molecular mass of the recombinant xylanase on SDS-PAGE was estimated to be 154 kDa, which agreed with the prediction from the amino acid sequence. The recombinant XynA was purified more than 73-fold, and the enzyme was obtained with a recovery yield of 38% (Table 2).
SDS-PAGE analysis of purifications of XynA from T. saccharolyticum NTOU1 in E. coli BL21(DE3) carrying the pET-23a-XynA a and zymogram analysis of xylanase activity b. Lane M protein marker, lane 1 crude extract from IPTG induced E. coli BL21(DE3) culture, lane 2 after affinity chromatography with a Ni Sepharose 6 Fast Flow column, lane 3 after gel filtration with Sephacryl S-300 column, lane 4 after anion exchange chromatography with Q Fast Flow column, and lane 5 zymogram of the purified enzyme. The gel was destained with 1 M NaCl until clear activity band against background
Characterization of the recombinant XynA
The effects of pH and temperature on the recombinant XynA are presented in Fig. 3. The enzyme was optimally active at pH 5.5 with more than 80% of the activity being preserved from pH 5.0 to 6.5 (Fig. 3a). XynA was stable between pH 6 and 7 retaining about 70% activity after 24 h (Fig. 3b). The optimum temperature for XynA was 72°C (Fig. 3c). The thermal inactivation was 34.2 kJ/mole calculated from Ln (specific activity) versus 1/temperature and the Arrhenius equation. Temperatures above 75°C sharply decreased the activity (Fig. 3d). The enzyme maintained activity at 65°C for 2 h indicating that it is thermostable (Fig. 3d). However, it lost activity after 20 min over 75°C (Fig. 3d).
Effects of temperature and pH on the activity and stability of the recombinant XynA. a The effect of pH on XynA activity. The relatively activity of 100% was 315 U/mg. b The effect of pH on stability. The enzyme was incubated at various pH levels at 25°C for 24 h, and the remaining activities were assayed at 72°C and pH 5.5. The relatively activity of 100% was 326 U/mg. c The effect of temperature on xylanase activity. The relatively activity of 100% was 324 U/mg. d The effect of temperature on stability. Purified XynA was incubated at temperatures 65°C (diamonds), 72°C (squares), 75°C (circles) and 80°C (triangles), respectively, without substrate (pH 5.5) for 0–120 min, and the remaining activities were assayed at 72°C and pH 5.5. The relatively activity of 100% was 335 U/mg. The error bars show the standard deviations from three measurements
Substrate specificity for the recombinant xylanase was determined using birch wood xylan (321 ± 8 U/mg), beech wood xylan (434 ± 16 U/mg), and oat spelt xylan (143 ± 7 U/mg). XynA was unable to degrade cellulose, carboxymethyl cellulose, starch or locust bean gum. In addition, XynA degraded xylotriose and xylopentaose, but not xylobiose (see Fig. 4). Therefore, XynA is able to degrade both xylan and short-chain xylooligosaccharides but not xylobiose. These results also were found for the GH10 xylanase of Thermoanaerobacterium saccharolyticum B6A-RI (Lee et al. 1993a).
Thin-layer chromatogram of hydrolyzation products of xylooligosaccharides by purified XynA. The enzyme reaction was carried out at 70°C and pH 5.5 for 2 h. The TLC plate was developed with a solvent containing of 1-butanol, 2-propanol, acetic acid, and water (7:5:2:4 v/v). After developing, the TLC plate was sprayed with solvent mix of aniline, diphenylamine, acetone, and 85% phosphoric acid (0.4:0.4:20:3 v/v). Spots were stained after heating at 100°C for 30 min. The standards (S) were xylose (X1), xylobiose (X2), xylotriose (X3), and xylopentaose (X5), respectively
Salt activation and salt-tolerance of XynA
Activity of XynA was enhanced by 0.1–0.5 M NaCl (Fig. 5a) with a 1.9-fold increase using 0.4 M NaCl. XynA maintained activity in the presence of 1 M NaCl (Fig. 5b) and 71% activity was retained after incubation with 2 M NaCl for 24 h. Salt-tolerant properties of XynA were similar to xylanases from a mesophilic Bacillus sp. NTU-06 (Wang et al. 2010), which were also isolated from salterns. Similar properties related to optimal temperature, pH, and thermostability were also observed in xylanases from T. saccharolyticum B6A-RI and Thermoanaerobacterium sp. JW/SL-YS 485 (Table 3). However, salt and chemical effects of these two enzymes were not reported. Our results show that XynA from T. thermosaccharolyticum has both thermophilic and salt-tolerant properties.
Salt activation and salt-tolerance of XynA. a Salt activation. The enzyme was assayed in the reaction mixtures containing different concentrations of NaCl (0 M to 0.5 M). The relatively activity of 100% was 327 U/mg. b Salt-tolerance. The enzyme was incubated with 0 to 2.0 M NaCl (pH 5.5) at room temperature for 24 h. The residual activity of XynA was assayed under standard conditions. The relatively activity of 100% was 319 U/mg. The error bars show the standard deviations from three measurements
In conclusion, the XynA gene was cloned from T. thermosaccharolyticum NTOU1 and over- expressed in E. coli. XynA consists of multiple domains including the glycoside hydrolase family 10 domains, carbohydrate-binding module domains, and surface layer homology domains. XynA is highly active in the temperature range from 70 to 73°C with an optimal temperature at 72°C. The enzyme retains good activity in a high salt concentration environment. The thermostable and salt-tolerant properties of XynA from T. saccharolyticum NTOU1 suggest that this enzyme has the potential to be used for industrial applications.
References
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Fan CL, Han XY, Xu ZR, Wang LJ, Shi LR (2009) Effects of beta-glucanase and xylanase supplementation on gastrointestinal digestive enzyme activities of weaned piglets fed a barley-based diet. J Anim Physiol Anim Nutr 93:271–276
Guo B, Chen XL, Sun CY, Zhou BC, Zhang YZ (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-beta-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84:1107–1115
Lee YE, Lowe SE, Zeikus JG (1993a) Gene cloning, sequencing, and biochemical characterization of endoxylanase from Thermoanaerobacterium saccharolyticum B6A-RI. Appl Environ Microbiol 59:3134–3137
Lee YE, Lowe SE, Zeikus JG (1993b) Regulation and characterization of cylanolytic enzymes of Thermoanaerobacterium saccharolyticum B6A-RI. Appl Environ Microbiol 59:763–771
Lin CJ, Tseng WC, Lin TH, Liu SM, Tzou WS, Fang TY (2010) Characterization of a thermophilic l-rhamnose isomerase from Thermoanaerobacterium saccharolyticum NTOU1. J Agric Food Chem. doi:10.1021/jf102063q
Liu SY, Gherardini FC, Matuschek M, Bahl H, Wiegel J (1996) Cloning, sequencing, and expression of the gene encoding a large s-layer-associated endoxylanase from Thermoanaerobacterium sp. strain JW/SL-YS 485 in Escherichia coli. J Bacteriol 178:1539–1547
Mesnage S, Fontaine T, Mignot T, Delepierre M, Mock M, Fouet A (2000) Bacterial slh domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J 19:4473–4484
Oren A (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol 28:56–63
Setati ME (2010) Diversity and industrial potential of hydrolase-producing halophilic/halotolerant eubacteria. Afr J Biotechnol 9:1555–1560
Shao W, Deblois S, Wiegel J (1995) A high-molecular-weight, cell-associated xylanase isolated from exponentially growing Thermoanaerobacterium sp. strain JW/SL-YS485. Appl Environ Microbiol 61:937–940
Tunnicliffe RB, Bolam DN, Pell G, Gilbert HJ, Williamson MP (2005) Structure of a mannan-specific family 35 carbohydrate-binding module: evidence for significant conformational changes upon ligand binding. J Mol Biol 347:287–296
Tuteja R (2005) Type I signal peptidase: an overview. Arch Biochem Biophy 441:107–111
van den Burg B (2003) Extremophiles as a source for novel enzymes. Curr Opin Microbiol 6:213–218
Wang CY, Chan H, Lin HT, Shyu YT (2010) Production, purification and characterisation of a novel halostable xylanase from Bacillus sp. NTU-06. Ann Appl Biol 156:187–197
Ziaie-Shirkolaee Y, Talebizadeh A, Soltanali S (2008) Comparative study on application of T. lanuginosus SSBP xylanase and commercial xylanase on biobleaching of non wood pulps. Bioresour Technol 99:7433–7437
Acknowledgments
This work was financially supported by the Center for Marine Bioscience and Biotechnology (CMBB). We thank Dr. Tze-Tze Liu for the consultation on whole genomic DNA sequencing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hung, KS., Liu, SM., Fang, TY. et al. Characterization of a salt-tolerant xylanase from Thermoanaerobacterium saccharolyticum NTOU1. Biotechnol Lett 33, 1441–1447 (2011). https://doi.org/10.1007/s10529-011-0579-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0579-7