Abstract
A hexavalent chromium [Cr(VI)] reducing bacterial strain was isolated from chromium-containing slag. It was identified as Pannonibacter phragmitetus based on physiological, biochemical characteristics and 16S rRNA gene sequence analysis. This bacterium displayed great Cr(VI) reduction capability. The Cr(VI) could be completely removed in 24 h under anaerobic condition when the initial concentration was 1,917 mg L−1, with the maximum reduction rate of 562.8 mg L−1 h−1. The Cr(VI) reduction rate increased with the increase of Cr(VI) concentration. P. phragmitetus was able to use many carbon sources such as lactose, fructose, glucose, pyruvate, citrate, formate, lactate, NADPH and NADH as electron donors, among which the lactate had the greatest power to promote the reduction process. Zn2+, Cd2+ and Ni2+ inhibited, while Cu2+, Pb2+, Mn2+ and Co2+ stimulated the reduction. The optimum pH and temperature for reduction were 9.0 and 30 °C, respectively. The results indicated that this strain had great potential for application in the bioremediation of chromate-polluted soil and water systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is an important heavy metal that is widely used in industrial processes such as ore refining, electroplating, production of steel and alloys, metal plating, tannery, wood preservation, pigmentation, etc. [1]. Untreated Cr(VI)-containing waste generated from the above processes was directly released into the environment and caused serious pollution. There are nine valency states of chromium ranging from −2 to +6 in nature, but only Cr(III) and Cr(VI) are of major environmental significance [2]. Cr(VI) is relatively more water soluble, bioavailable, reactive and toxic than Cr(III). Compounds containing Cr(VI) were reported as mutagenic, carcinogenic and teratogenic [3–5]. Cr(III) is thermodynamically stable and less toxic. It is also an essential micronutrient for proper glucose metabolism that stimulates the enzyme system and stabilizes nucleic acids [6]. Consequently, the reduction of toxic Cr(VI) to stable Cr(III) is considered as an efficient way to recover chromate pollution from soil and water systems. In addition, Cr(III) is easily formed as precipitate Cr(OH)3 or Cr2O3 [7].
Detoxification and removal of Cr(VI) through reduction of Cr(VI) to Cr(III) can be carried out with physicochemical or biological methods. The conventional physicochemical treatment technologies include ion exchange, chemical reduction, adsorption, precipitation and electrodialysis [8]. However, these methods consume high amounts of energy and large quantities of chemical reagents and therefore are not economically feasible. Furthermore, the resultant metal-containing chemical sludge is a potential source of metal pollution [9]. Alternatively, biological processes for treating chromium-contaminated sites are becoming very promising because of the high efficiency, low operating cost, short operation time and eco-friendliness [10].
Bioremediation of Cr(VI) by microorganisms has emerged as a potential alternative for detoxification and recovery of toxic and valuable metals from polluted environments. Three microbial Cr(VI) reduction mechanisms have been described: Chromate reduction under aerobic conditions is commonly associated with soluble reductases that use NADH or NADPH as cofactors; Cr(VI) was used as an electron acceptor in the electron transport chain under anaerobic conditions; Cr(VI) may also be reduced by unspecific reactions associated with organic compounds such as amino acids, nucleotides, sugars, etc. [11]. Various bacteria capable of converting Cr(VI) into less toxic Cr(III) have been identified under both aerobic and anaerobic conditions, including Achromobacter sp. [12], Bacillus sp. [13, 14], Providencia sp. [15], Pseudomonas aeruginosa [16], Burkholderia cepacia [17], Escherichia coli [18, 19], etc. However, the Cr(VI) reduction efficiency of these strains were not high enough.
In a previous study, we have reported a strain having the ability for Cr(VI) reduction. It was identified as P. phragmitetus [1]. P. phragmitetus strain had not been characterized as being able to reduce Cr(VI) previously (to our knowledge). In the present study, another strain stored in our laboratory was carefully investigated. It was also identified as P. phragmitetus and had much stronger Cr(VI) reducing power.
Materials and methods
Bacterial strain and cultural conditions
The Cr(VI) reducing strain was isolated from the soil collected from the slag sites of chromate ore processing in Changsha, China and stored in the School of Metallurgical Science and Engineering, Central South University, China. Cells were grown in the nutrient medium containing 5 g tryptone, 2 g NaCl, 5 g yeast extract and 5 g sodium lactate in 1 L distilled water (the contents of K2Cr2O7 was properly adjusted according to experimental requirement) at pH 9 with shaking speed of 150 rpm. All media were autoclaved at 121 °C for 25 min before use.
Identification of the strain reducing Cr(VI)
Genomic DNA was extracted and purified using the TIANamp Bacteria DNA Kit (TIANGEN China). The 16S rRNA was amplified from chromosomal DNA of Cr(VI) reduced strain by PCR. The primers were 27F and 1492R [20]. The PCR amplification was performed as follows: each reaction was performed in a final volume of 50 μL, containing 25 μL 2× mix, 1 μL each primer, 1 μL DNA sample and 22 μL deionized water. The reaction mixture was subjected to 30 cycles of amplification, denaturation at 94 °C for 30, annealing at 55 °C for 60 s and extension at 72 °C for 60 s. PCR products were purified using the TIAquick Midi Purification Kit (TIANGEN China) and sent to Shanghai Biological Company for sequencing. The sequence was aligned with that of other bacterial species obtained from the GenBank database and corresponding sequences were downloaded. CLUSTALX program was used to align bacteria nucleotide sequences and construct phylogenetic tree. Physiological and biochemical characteristics of the strain reducing Cr(VI) were performed using the methods as described previously [21].
Preparation of cell suspension
The P. phragmitetus strain that grew for about 12 h in 200 mL of liquid nutrient medium was harvested by centrifugation at 10,000 rpm for 10 min at 4 °C, washed twice with Tris–HCl (100 mmoL L−1, pH 9.0) and resuspended in 200 mL with the same buffer. Flasks containing cell suspensions were sealed with butyl rubber stoppers and purged with N2 for 20 min to free O2 and gain an anaerobic condition. Cell concentration expressed as OD600 was retained to 1.27. Then, the cell suspension was placed in flasks and stored at 4 °C in a refrigerator before use.
Cr(VI) reduction experiments
The reaction mixtures were set up in 40-mL sealed serum bottles, and the final volume was 20 mL. Cr(VI) reduction studies were started by the addition of Cr(VI) (200 mg L−1) under anaerobic condition. Samples were withdrawn at intervals by a sterile syringe, and supernatants were analyzed for residual Cr(VI).
To characterize the Cr(VI) reduction efficiency of P. phragmitetus, the effect of temperature (20, 25, 30, 35, 40, 45 and 50 °C), pH (6, 7, 8, 9, 10, 11 and 12), initial cell concentration (0.059 × 109 to 3.41 × 109 cells mL−1) and initial Cr(VI) concentration (104–2,031 mg L−1) were investigated via resting cells or growing cells. Cr(VI) reduction was studied under anaerobic condition in 40-mL sealed serum bottles with a 20-mL mixture. The mixture was obtained from the suspension (2% sodium lactate was added) prepared above or from log bacterial culture with the desired concentration of cells and supplemented with appropriate amount of Cr(VI). It was incubated at the optimal temperature and pH with shaking condition (150 rpm). Samples were drawn at definite time intervals, centrifuged at 10,000 rpm for 10 min and the supernatant fluid was analyzed for residual Cr(VI). All the experiments were done in triplicate.
The effect of different carbon sources (2%) including lactose, fructose, glucose, pyruvate, citrate, formate, lactate, NADPH and NADH on Cr(VI) reduction was investigated in cell suspension containing 200 mg L−1 Cr(VI). Cell suspension with 200 mg L−1 Cr(VI) and no carbon source was set as control. The effect of electron donors on Cr(VI) reduction was also investigated in cell suspension containing 200 mg L−1 Cr(VI), 2% sodium lactate and one of the following heavy metals: 20 mg L−1 (Co2+, Cd2+) and 100 mg L−1 (Cu2+, Mn2+, Ni2+, Pb2+, Zn2+). The cell suspension with 200 mg L−1 Cr(VI), 2% sodium lactate and no heavy metal was set as control. All cell suspensions were incubated at the optimal temperature and pH with shaking (150 rpm). All the experiments were done in triplicate.
Analysis methods
Samples were withdrawn periodically, via a syringe, and the decrease in chromate concentration in supernatant with time was estimated using the Cr(VI)-specific colorimetric reagent S-diphenylcarbazide (DPC), which was prepared in acetone/H2SO4 to minimize deterioration as described previously [22]. Cell density was determined following previous work [12]. Reduction rates were designated as the amount of Cr(VI) reduced per hour (mg L−1 h−1). Cell suspensions used in the above experiments were of original concentration, approximately 3.41 × 109 cells mL−1 (OD600 = 1.27), and the ratio of cell to Cr(VI) was 1.1 × 1010 cells mg−1.
Droplets of a small amount of liquid mixture from different stages of Cr(VI) reduction were placed on glass slides. After 15 min adsorption, glutaraldehyde (2.5%) was added for immobilization for 1 h. Then the sample was dehydrated in gradient using 30–70% ethanol and replaced by isoamyl acetate for 30 min. Through critical point drying (HITACHI HCP-2 Critical PointDryer) and ion sputtering (Eiko IB-3 ion plating machine), the sample was observed via scanning electron microscopy (SEM) (JEOL JSM-6360LV).
Results and discussion
Identification of Cr(VI) reducing strain
The bacterial strain with high ability of reducing Cr(VI) was selected for identification. Its physiological and biochemical characteristics were basically similar to P. phragmitetus (Table 1). The 16S rRNA sequence size was 1,388 bp and displayed over 99% similarity with that of P. phragmitetus. The partial 16S rRNA gene sequence of the microorganism has been deposited in GenBank with the accession number JN626199. The phylogenetic tree (Fig. 1) showed that the stain tightly clustered with P. phragmitetus. Accordingly, the strain was indentified as P. phragmitetus.
Phylogenetic tree obtained from 16 SrRNA sequence comparisons 1,355 bp bases showing the relationship between members of the family Pannonibacter and the strain stored in our laboratory. The bootstrap neighbor-joining tree (random number generator seed = 57, trails = 1,000) was constructed with Clustal X version 2.0. GeneBank accession numbers are in brackets. Scale bar 0.01 base differences per position. 1 Previously reported strain; 2 the first reported Pannonibacter phragmitetus strain [24]; 3 the Cr(VI) reducing strain in the present study
Effects of temperature and pH on Cr(VI) reduction
Temperature is an important factor affecting biological Cr(VI) reduction. Cr(VI) reduction by P. phragmitetus was evaluated under seven different temperatures, namely, 15, 20, 25, 30, 35, 40 and 45 °C. The Cr(VI) was reduced fairly well (31.7–99.1%) from 15 to 45 °C with the optimum at 30 °C (Fig. 2a). However, the optimum temperature for growth was 35 °C (Fig. 2b), where the Cr(VI) removal was 96.9%. It is indicated that Cr(VI) reduction would be inhibited if the temperature is too high or low. Therefore, if the strain was used for chromium pollution recovery, the temperature should be controlled at 30–35 °C. As reported, a wide range of temperature from 10 to 40 °C was recorded for Cr(VI) reduction by Enterobacter cloacae strain HO1 with the optimum of 30 °C [25]. Bacterial growth and microbial Cr(VI) reduction of strain DM1 were investigated under 30–45 °C. The result showed that both the optimal growth temperature and Cr(VI) reduction temperature were 35 °C [26].
The variation of Cr(VI) concentration under different pH values of 6–12 is shown in Fig. 2c. The Cr(VI) was reduced when the pH ranged from 7 to 11. There was no obvious difference in Cr(VI) reduction at pH 9 and 10, but Cr(VI) reduction almost ceased at pH 6 and 12. The optimum value was 10. However, the optimum pH for growth was 8 (Fig. 2d). In addition, with the change of Cr(VI) concentration, the pH value of all media had a trend of change to about 8 (data not shown). The result indicated that the operation of P. phragmitetus reducing Cr(VI) mechanism may be priority to other physiological mechanisms to ensure the bacterial growth in the environment with high chromium content. In a previous report, the optimal pH was also 9 for Cr(VI) reduction by Gram-positive bacterium [27] and Ochrobactrum sp. strain CSCr-3 [28].
Effects of different electron donors on Cr(VI) reduction
During the process of reduction, the Cr(VI) was converted into Cr(III) via accepting three electrons. Consequently, there must be electron donor(s) to provide electrons. A variety of organic compounds were utilized by P. phragmitetus as electron donors for Cr(VI) reduction. In the resting cell suspension, when lactose, fructose, glucose, pyruvate, citrate, formate, lactate, NADPH and NADH were used as carbon source, the Cr(VI) reduction activity was 30, 20, 7, 78, 5, 17, 123, 70 and 90%, respectively. Those values were more than that of the control (Fig. 3). The result was consistent with previous report that Streptomyces griseus NCIM2020 was capable of using many substrates including glucose, sucrose, acetate, citrate, tartrate, glycerol and ethanol as electron donors, and the Cr(VI) reduction activity was also increased in varying degrees [29]. Electron donors provide electrons for Cr(VI) reduction. The varying degrees of chromium reduction activity under various electron donors might be probably because the electron-accepting ability of reductase enzymes for different electron donors was different.
Effects of initial cell concentrations and chromate concentrations on Cr(VI) reduction
Effects of initial bacterial cell concentrations (0.059 × 109 to 3.41 × 109 cells mL−1) on Cr(VI) (250 mg L−1) reduction are normally distributed (Fig. 4a). Accordingly, Cr(VI) reduction rates were equal to the slope of the line. From the lowest cell concentration (0.059 × 109 cells mL−1) to the highest cell concentration (3.41 × 109 cells mL−1), the Cr(VI) was completely reduced within 9.5, 6, 4, 2.5 and 2 h, respectively, and the reduction rates increased from 26.5 to 125.0 mg L−1 h−1. Cr(VI) reduction by P. phragmitetus increased with the increase in initial cell concentrations from 0.059 × 109 to 3.41 × 109 cells mL−1 as observed by other researchers [30, 31].
Effects of initial chromate concentrations on Cr(VI) reduction by growing and resting cell (3.41 × 109 cells mL−1) of P. phragmitetus are shown in Fig. 4b, c. The initial rate of Cr(VI) reduction by growing cell increased with the increase of chromate concentration from 100 to 700 mg L−1. When the chromate concentration was up to 900 mg L−1, the initial rate was decreased. In addition, the higher the chromate concentration, the lower was the initial rate. This may be because the high concentration of Cr(VI) would adversely affect the growth of cells. But through a period of adaptation, the reduction rate increased to a relatively high value and then decreased. This may be attributed to the pH change (“Effects of temperature and pH on Cr(VI) reduction”) or cell degradation at the later stage of the reaction. The maximum Cr(VI) reduction rate and the largest reduction capacity by growing cell were up to 332.0 and 1,791 mg L−1, respectively. Cr(VI) reduction by resting cell was different. The initial rate of Cr(VI) reduction increased with the increase of chromate concentrations from 300 to 2,031 mg L−1, but decreased with the change of Cr(VI) concentrations for the same reason of pH change (“Effects of temperature and pH on Cr(VI) reduction”). The maximum Cr(VI) reduction rate and the largest reduction capacity were up to 562.8 mg L−1 h−1 and 1,917 mg L−1, respectively. The maximum Cr(VI) reduction rate and the largest reduction capacity of resting cells are all higher than those of growing cells; Cr(VI) concentration has little or no adverse effect on resting cells because these are not growing.
The Cr(VI) reduction efficiency by P. phragmitetus is very high. The maximum Cr(VI) reaction rate and the largest reduction capacity were both higher than those reported by other researchers [5, 30–32].
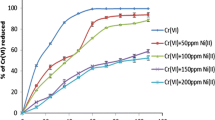
Effects of heavy metals on Cr(VI) reduction
As other metals also existed in industrial effluents, the effects of other heavy metal cations on Cr(VI) reduction by P. phragmitetus were also investigated (Fig. 5). The presence of Cu2+, Pb2+, Mn2+ and Co2+ significantly enhanced Cr(VI) reduction by 21, 12, 16 and 7%, respectively, while Zn2+, Cd2+ and Ni2+ inhibited Cr(VI) reduction by 46, 28 and 14%, respectively. Thus, Cu2+ and Zn2+ showed the highest stimulatory and inhibitory effect on Cr(VI) reduction, respectively. Stimulatory effects of Cu2+, Mn2+ and Co2+ on Cr(VI) reduction were also reported with Ochrobactrum sp. strain CSCr-3 [28] and Ochrobactrum intermedium strain SDCr-5 [30]. However, the inhibition of Cu2+ on Cr(VI) reduction by many other microorganism such as Escherichia coli [19, 31], etc., and the inhibitions of Pb2+ and Co2+ on Cr(VI) reduction by Bacillus sphaericus were also reported [33]. The mechanism of the effect of the heavy metals on Cr(VI) reduction is still unclear. As known, Cu2+ is the prosthetic group for many reductase enzymes. The main function of Cu2+ has been reported to be related to electron transport protection or acting as electron redox center and, in some cases, as a shuttle for electrons between protein subunits [34]. It was presumed that other stimulatory effect metals may have the same mechanism. Inhibitory effect metals may change pH or combine with functional groups of reductase enzymes during Cr(VI) reduction. Further studies of a reasonable mechanism are needed.
Bacterial cell morphology during the process of Cr(VI) reduction and reduction products
To further understand Cr(VI) reduction by P. phragmitetus at the macroscopic level, the resting cells of P. phragmitetus in three different Cr(VI) reduction periods were collected for SEM observation. As shown in Fig. 6, the distribution of resting cells was relatively uniform in buffer in the initial stage (Fig. 6a); in the middle stage, it was obviously observed that the resting cells agglomerated and reduced Cr(VI) synergistically (Fig. 6b); and in the last stage, resting cells became transparent, and the distribution was scattered again (Fig. 6c). The results indicated that Cr(VI) reduction by P. phragmitetus was a process of synergistic effect and that the reaction might be carried out more efficiently when the resting cells agglomerated. This gave an explanation for the above conclusion that Cr(VI) reduction was increased with an increase in the initial cell concentration (3.4).
During the process of Cr(VI) reduction by P. phragmitetus, the color of the reaction mixture changed from yellow to blue, and a large quantity of dark blue precipitate was formed. In our previous work [35], we have proved that the main components of the precipitate from Cr(VI) reduction by P. phragmitetus were chromium compounds. In addition, other microorganisms such as Achromobacter sp. [12] Cellulomonas spp. [36], etc. were reported to be able to produce chromium compounds during the process of Cr(VI) reduction.
Conclusions
A bacterial strain identified as P. phragmitetus was proved to be able to effectively reduce Cr(VI). Higher initial cell and Cr(VI) concentration and addition of lactate as carbon sources increased the Cr(VI) reduction ability of P. phragmitetus. The process of the reduction was enzymatic, so purification and characterization of chromate reductase is under way. Biological reduction of Cr(VI) by this strain can be used as an efficient and eco-friendly technique for Cr(VI) pollution control. Consequently, further understanding of the bacterial Cr(VI) reduction mechanism is necessary.
References
Chai LY, Huang SH, Yang ZH, Peng B, Huang Y, Chen YH (2009) Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J Hazard Mater 167:516–522
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Coata M (2003) Potential hazards of hexavalent chromate in our drinking water. Toxicol Appl Pharmacol 1:1–5
Garbisu C, Alkorta I, Llama Maria J, Serra JL (1998) Aerobic chromate reduction by Bacillus subtilis. Biodegradation 9:133–141
McLean J, Beveridge TJ (2001) Chromate reduction by a pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084
Viti C, Pace A, Giovannetti L (2003) Characterization of Cr(VI)-resistant bacteria isolated from chromium-contaminated soil by tannery activity. Curr Microbiol 46:1–5
Puzon GJ, Roberts AG, Kramer DM, Xun L (2005) Formation of soluble organo-chromium(III) complexes after chromate reduction in the presence of cellular organics. Environ Sci Technol 39:2811–2817
Krishna RK, Philip L (2005) Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater 20:109–117
Pei QH, Shahir S, Santhana Raj AS, Zakaria ZA, Ahmad WA (2009) Chromium(VI) resistance and removal by Acinetobacter haemolyticus. World J Microbiol Biotechnol 25:1085–1093
Srividya K, Mohanty K (2009) Biosorption of hexavalent chromium from aqueous solutions by Catla catla scales: equilibrium and kinetics studies. Chem Eng J 155:666–673
Cervantes C, Campos-García J (2007) Reduction and efflux of chromate by bacteria. Microbiol Monogr 6:407–419
Zhu WJ, Chai LY, Ma ZM, Wang YY, Xiao HJ, Zhao K (2008) Anaerobic reduction of hexavalent chromium by bacterial cells of Achromobacter sp. strain Ch1. Microbiol Res 163:616–623
Liu YG, Xu WH, Zeng GM, Li X, Gao H (2006) Cr(VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem 41:1981–1986
Cheng GJ, Li XH (2009) Bioreduction of chromium(VI) by Bacillus sp. isolated from soils of iron mineral area. Eur J Soil Biol 45:483–487
Thacker U, Parikh R, Shouche Y, Madamwar D (2006) Hexavalent chromium reduction by Providencia sp. Process Biochem 41:1332–1337
Xu WY, Liu YG, Zeng GM, Li X, Song HX, Peng QQ (2009) Characterization of Cr(VI) resistance and reduction by Pseudomonas aeruginosa. Trans Nonferrous Met Soc China 19:1336–1341
Wani R, Kodam KM, Gawai KR, Dhakephalkar PK (2007) Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline crater lake. Appl Microbiol Biotechnol 75:627–632
Bae WC, Lee HK, Choe YC, Jahng DJ, Lee SH, Kim SJ, Lee JH, Jeong BC (2005) Purification and characterization of NADPH-dependent Cr(VI) reductase from Escherichia coli ATCC 33456. J Microbiol 43:21–27
Liu GF, Yang H, Wang J, Jin RF, Zhou JT, Lv H (2010) Enhanced chromate reduction by resting Escherichia coli cells in the presence of quinone redox mediators. Bioresour Technol 101:8127–8131
Polz MF, Cavanaugh CM (1998) Bias in template to product ratios in multitemplate PCR. Appl Environ Microbiol 64:3724–3730
Qian CR, Huang YX (2008) Laboratory experiments in microbiology. Beijing University press, Beijing
Pattanapipitpaisal P, Brown N, Macaskie L (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI) contaminated site. Appl Microbiol Biotechnol 57:257–261
Georgy GM, Julia BA, Timothy LG (2004) Bergery’s manual of systematic bacteriology. Springer, New York
Borsodi AK, Micsinai A, Kovács G, Tóth E, Schumann P, Kovács AL, Böddi B, Márialigeti K (2003) Pannonibacter phragmitetus gen. nov., sp. nov., a novel alkali-tolerant bacterium isolated from decomposing reed rhizomes in a Hungarian soda lake. Int J Syst Evol Microbiol 53:555–561
Wang PC, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H (1989) Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol 55:1663–1670
Urvashi T, Datta M (2005) Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microb Biot 21:891–899
Shakoori AR, Makhdoom M, Haq RU (2000) Hexavalent chromium reduction by a dichromate-resistant gram-positive bacterium isolated from effluents of tanneries. Appl Microbiol Biotechnol 53:348–351
He ZG, Gao FL, Sha T, Hu YH, He C (2009) Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater 163:869–873
Poopal AC, Laxman RS (2009) Studies on biological reduction of chromate by Streptomyces griseus. J Hazard Mater 169:539–545
Sultan S, Hasnain S (2007) Reduction of toxic hexavalent chromium by Ochrobactrum intermedium strain SDCr-5 stimulated by heavy metals. Bioresour Technol 98:340–344
Shen H, Wang YT (1994) Biological reduction of chromium by E. coli. J Environ Eng 120:560–570
Xu L, Luo MF, Li WL, Wei XT, Xie K, Liu LJ, Jiang CY, Liu HZ (2011) Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater 185:1169–1176
Pal A, Paul AK (2004) Aerobic chromate reduction by chromium resistant bacteria isolated from serpentine soil. Microbiol Res 159:347–354
Abe F, Miura T, Nagahama T, Inoue A, Usami R, Horikoshi K (2001) Isolation of a highly copper-tolerant yeast, Cryptococcus sp., from the Japan trench and the induction of superoxide dismutase activity by Cu2+. Biotechnol Lett 23:2027–2034
Chai LY, Huang SH, Yang ZH, Peng B, Huang Y, Chen YH (2009) Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium-containing slag heap. J Environ Sci Health A Tox Hazard Subst Environ Eng 44:615–622
Viamajala S, Smith WA, Sani RK, Apel WA, Petersen JN, Neal AL, Roberto F, Newby D, Peyton BM (2007) Isolation and characterization of Cr(VI) reducing Cellulomonas spp. from subsurface soils: implications for long-term chromate reduction. Bioresour Technol 98:612–622
Acknowledgments
This work was funded by the Major Program of Water Pollution Control and Treatment of China (2009zx07212-001-01) National Funds for Distinguished Young Scientists (50925417) and National Natural Science Foundation of China (51074191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Y., Chai, L., Yang, Z. et al. Identification and hexavalent chromium reduction characteristics of Pannonibacter phragmitetus . Bioprocess Biosyst Eng 35, 843–850 (2012). https://doi.org/10.1007/s00449-011-0668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0668-y