Abstract
The Cr(VI)-reducing bacterial strain MCMB-821 was isolated from the alkaline crater lake of Lonar and was identified as Burkholderia cepacia. MCMB-821 was resistant to 1,000-ppm Cr(VI) and reduced 98% of the 75 ppm Cr(VI) within 36 h at pH 9.0 in the presence of 2% salt and lactose as the electron donor. The chromate-reducing efficiency of MCMB-821 was comparable under both aerobic as well as anaerobic conditions. Electron paramagnetic resonance spectroscopy data suggested that MCMB-821 reduced Cr(VI) to Cr(III) via the formation of transient Cr(V) intermediate. The chromate-reducing ability of MCMB-821 was suppressed in the presence of membrane inhibitors and enhanced in the presence of 2,4-dinitrophenol, suggesting the involvement of electron transport chain in the Cr(VI) bioreduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium, a steel gray lustrous brittle metal occurs in several oxidation states ranging from (−II) to (+VI), trivalent and hexavalent states being the most stable ones (Nieboer and Jusys 1988). Chromium in hexavalent form is highly soluble and bioavailable (Yadav et al. 2005). The carcinogenic, clastogenic, and teratogenic potential of Cr(VI) is attributed to their strong oxidizing property (Yassi and Nieboer 1988; USEPA 1998; Chardin et al. 2002). The trivalent chromium on the contrary is immobile and less soluble because it precipitates out as a strong oxide and hydroxide above pH 5.0, rendering it less toxic and less bioavailable (Mclean and Beveridge 2000; Upreti et al. 2004). Hexavalent chromium, an EPA priority pollutant, is a predominant waste product of several metal finishing, tanning, petroleum refining, and iron and steel industries. It exists primarily as either chromate (\( {\text{CrO}}^{{2 - }}_{4} \)) in basic and neutral environments or as dichromate (\( {\text{Cr}}_{{\text{2}}} {\text{O}}^{{2 - }}_{7} \)) in acidic environment (Yadav et al. 2005; Middleton et al. 2003; Pal and Paul 2004).
The conventional metal cleanup technologies like soil removal and land filling, stabilization, physico-chemical extraction, and soil washing (Jeyasingh and Philip 2005) offer a temporary solution by simply immobilizing the contaminant (Pal and Paul 2004). Also, chemical methods of the reduction of chromate are prohibitively expensive for large-scale decontamination (Ackerley et al. 2004). They have been also believed to be responsible for the formation of huge quantities of metal containing chemical sludge that poses additional problem of disposal (Thacker and Madamwar 2005). Owing to the aforementioned difficulties, bioremediation strategy for heavy metal detoxification has wider implications. Hence, for effective, economic waste remediation, further exploration in bioremediation technology is required. This technique serves to detoxify Cr(VI) to the trivalent state, thereby immobilizing it in the soil matrix. Besides eliminating the toxicity, the trivalent species forms an insoluble Cr(OH)3 precipitate, severely debilitating its migration to groundwater (Jeyasingh and Philip 2005).
Many bacterial species have been reported to reduce Cr(VI) under aerobic or anaerobic conditions, most of which have been isolated from indigenous microbial population (Mclean and Beveridge 2000; Elangovan et al. 2006). However, reports on the occurrence of Cr(VI)-reducing strains from a natural ecosystem have been relatively few (Pal and Paul 2004). In the present investigation, we have reported a Cr(VI)-reducing bacterial strain (MCMB-821) isolated from the pristine habitat of alkaline soda lake created by meteorite impact at Lonar in Maharashtra state, India. The objective of the present study was to determine the ability of this alkaliphilic strain to reduce high concentration of Cr(VI) to Cr(III), optimize process parameters viz pH, temperature, salinity, cell density, effect of aerobic and anaerobic conditions, electron donors, time course, and finally, to determine the effect of membrane transport inhibitors on the Cr(VI) bioreduction.
Materials and methods
Nutrient media
The bacterial isolate (MCMB-821) was cultured aerobically at 35°C in alkaline nutrient medium (pH 9.0) with lactose (1 g/l) as the electron donor (Kodam et al. 2005). The culture was maintained on nutrient agar (Hi Media, Mumbai, India) slants at 4°C as well as glycerol stocks at −20°C.
Hexavalent chromium analysis
Hexavalent chromium was estimated by diphenyl carbazide (DPC) method as described in APHA–AWWA–WPCF (1985). Chromium concentration was determined using the standard calibration curve for the same.
Optimization of parameters
The alkaliphilic nutrient media was amended with 75-ppm Cr(VI) to determine the effect of varying pH on Cr(VI) reduction, ranging from 7 to 13. The reduction was monitored at regular intervals, and all the experiments were repeated in triplicates. On similar lines, the optimum temperature of MCMB-821-mediated Cr(VI) reduction was determined by evaluating the Cr(VI) reduction at 25, 30, 35, and 40°C. The effect of salt concentration on the Cr(VI) reduction efficiency of MCMB-821 was determined in the presence of NaCl (1 to 5%) in a alkaliphilic nutrient medium. MCMB-821 strain was also subjected to several electron donors (1%) such as glucose, sucrose, starch, lactose, sodium acetate, methanol, and ethanol to determine the most effective donor aiding this microbial reduction process. The hexavalent chromium-reducing efficiency of MCMB-821 was studied under aerobic and anaerobic conditions (nitrogen-flushed medium, incubated under nitrogen environment) at 35°C.
Concentration studies
The alkaliphilic nutrient broth was amended with variable concentration of Cr(VI), respectively 75, 200, 300, 400, 500, and 1,000 ppm to study the time duration required for the reduction of Cr(VI). Also, the maximum concentration of Cr(VI) reduced was determined at the end of a specified duration.
Electron paramagnetic resonance
The electron paramagnetic resonance (EPR) spectrum was recorded using a Brucker EMX EPR spectrometer. The spectral parameters were 100-kHz field modulation, 1.0-G modulation amplitude, 9.76-gHz microwave frequency, 4.01-mW power, and 2.5 modulation voltage. The reaction was carried out at room temperature.
Membrane transport inhibitors
To examine the possible involvement of membrane electron transport chain (ETC) in the bioreduction process, studies were conducted using several ETC inhibitors viz azide (1 mM), cyanide (3 mM), rotenone (1 mM), antimycin A (1 mM), and 2,4-dinitrophenol (DNP, 1 mM). These inhibitors were added to the Cr(VI)-amended nutrient medium, and their effect on reduction efficiency was determined after periodic withdrawal of aliquots for Cr(VI) analysis.
Statistical analysis
The average values of the minimum three independent experiments are described in this article. Statistical analysis was performed using Microcal origin-6 software (Northampton, USA).
Results
Characterization of the bacterial isolate
MCMB-821 was isolated from the sediment sample collected from the alkaline crater lake of Lonar in Maharashtra state, India. This alkaliphilic isolate was identified as Burkholderia cepacia by morphological and biochemical characteristics in accordance with “Bergey’s Manual of Systematic Bacteriology” (Kreig and Holt 1984) and also by API20NE system (%id 99.9%).
Effect of initial chromium concentration on Cr(VI) bioreduction
MCMB-821 was resistant to 1,000 ppm of Cr(VI) in the nutrient medium at 35°C. The chromate-reducing ability of MCMB-821 was monitored at different initial concentrations of Cr(VI) ranging from 75 to 500 ppm. MCMB-821 reduced more than 98% of the hexavalent chromium within 36 h when the initial concentration was 75 ppm (data not shown). However, the chromate-reducing efficiency of MCMB-821 decreased with a proportionate increase in the initial concentration of Cr(VI). MCMB-821 reduced 73, 67, and 53% of the 300, 400, and 500 ppm chromate, respectively, within 192 h when incubation was continued beyond 48 h up to 192 h (data not shown). The total cell protein of MCMB-821 increased linearly with an increase in the reduction of Cr(VI) as monitored periodically for up to 36 h for an initial concentration of 75 ppm, suggesting the enhancement in the reductase activity in response to Cr(VI) (Fig. 1).
Evaluation of optimum conditions
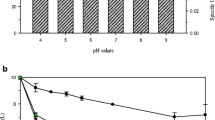
The optimum pH for MCMB-821-mediated Cr(VI) reduction was pH 9.0 (Fig. 2a). Nonetheless, MCMB-821 was also capable of reducing Cr(VI) at a wider pH range of 7–10 with an appreciable efficiency on either side of the pH optima. The bioreduction of hexavalent chromium was studied within a temperature range of 25 to 40°C. The optimum temperature for the growth of MCMB-821 was found to be 35°C. Similarly, the optimum temperature for the MCMB-821-mediated Cr(VI) reduction was also 35°C. The chromate reduction efficiency of MCMB-821 decreased on either side of temperature optima (Fig. 2b). MCMB-821 was also tested for its ability to reduce Cr(VI) in the presence of increasing salt concentration. It was observed that 2% salt concentration supported maximum reduction of hexavalent chromium (Fig. 2c). However, significant Cr(VI) reduction was observed in the presence of 1 to 5% salt concentration. The chromate reduction efficiency of MCMB-821 was found to increase with the increase in the cell density. Highest Cr(VI) reduction efficiency was noted when cell density was 108 cells/ml (Fig. 2d). The bioremediation of Cr(VI) was studied under aerobic and anaerobic conditions with an initial Cr(VI) concentration of 75 ppm. It was observed that under anaerobic conditions, the reduction of Cr(VI) within 48 h was almost 100% as compared to 97% reduction under aerobic conditions (data not shown).
Effect of electron donors
MCMB-821 efficiently reduced Cr(VI) in the presence of various electron donors such as lactose, glucose, sucrose, and starch (Fig. 3). However, the chromate reduction efficiency of MCMB-821 decreased when ethanol, methanol, and sodium acetate were used as electron donors. MCMB-821 reduced 97% of the Cr(VI) in less than 48 h when lactose (1%) was used as electron donor.
Electron paramagnetic resonance
In the present investigation, an EPR signal of g value 1.978 demonstrated a line width of 14.3 G indicating the presence of Cr(V), whereas the EPR signal of Cr(III) was centered at g 2.03 and had the line width of 1,875 G (Fig. 4).
Effect of membrane inhibitors
MCMB-821-mediated chromate reduction was monitored in the presence of several inhibitors of ETC to investigate the involvement of ETC in the chromate reduction process. It was observed that chromate reduction was inhibited in the presence of rotenone, antimycin A, and cyanide (Table 1). The chromate reduction efficiency of MCMB-821 was increased in the presence of DNP.
Discussion
MCMB-821 was isolated from the sediments of the alkaline crater lake at Lonar. It was identified as B. cepacia on the basis of biochemical and morphological characteristics, as well as by the API20NE system. Chromium-resistant bacteria capable of reducing chromate have been reported from chromium-polluted environments (Pal and Paul 2004; Camargo et al. 2003). This is an indication of the adaptation of the microorganisms to the toxic levels of Cr(VI) in the environment. However, the high level of chromium resistance displayed by the strain MCMB-821 was interesting, considering that the natural habitat of this isolate was not known to be contaminated with chromium. MCMB-821 could tolerate Cr(VI) up to 1,000 ppm. This observation is of significance from the standpoint of in situ bioremediation because it indicates the ability of MCMB-821 to withstand shock loads of Cr(VI) during the bioremediation process.
Majority of the hexavalent chromium-reducing species reported so far were Pseudomonas and were able to reduce Cr(VI) either at pH near neutrality (7–7.8) or in the acidic range (Liu et al. 2004; Losi et al. 1994). Wang et al. (1990) reported Cr(VI) reduction by Enterobacter cloacae at pH 6.5–8.5 and also that the reduction was strongly inhibited at pH 5.0 and 9.0. The bacterial strains, as reported by Jeyasingh and Philip (2005), maximally reduced 50 mg/l of the initial Cr(VI) at neutral pH and efficiently only under aerobic conditions. Similarly, Bacillus sphaericus isolated by Pal and Paul (2004) reduced 62% of the 20-mg Cr(VI)/l in 48 h. Bacillus sp. RE reported by Elangovan et al. (2006) reduced 95% of the 40-mg Cr(VI)/l in 72 h and reduced only 50% of the 80 mg/l of Cr(VI) under similar conditions. The optimum pH for the B. cepacia MCMB-821-mediated chromate reduction was found to be 9.0 for 75 mg/l of Cr(VI) within 36 h. This observation is of significance in deploying MCMB-821 for soil bioremediation because Cr(VI) is known to desorb from soil at a faster rate at elevated pH values (Wang et al. 2002). The ability of MCMB-821 to reduce chromate even at high salt concentration (up to 5%) suggested that MCMB-821 is a halotolerant culture and, therefore, may be a member of the haloalkaliphilic family. MCMB-821 reduced chromate at an efficiency of 98% or more under both aerobic and anaerobic conditions. This positively enhances its potential for bioremediation under variable environmental conditions.
Bacteria often preferentially utilize energetically more favorable electron donors. MCMB-821 efficiently reduced Cr(VI) in the presence of various electron donors such as lactose, glucose, sucrose, starch, sodium acetate, methanol, and ethanol. The efficiency of chromate reduction was variable with different electron donors. This was suggestive of a certain metabolite of sugar being utilized as an actual electron donor for the electron-deficient hexavalent species because the metabolism of sugars into reductant may have been limited by other substrate or cofactors in the cell. The efficacy of bioreduction by MCMB-821 in the presence of several electron donors also elucidates its non-fastidious nature, thereby encouraging its range of application because several substances present in soils with variable composition may serve as electron donors for Cr(VI) reduction mediated by MCMB-821.
EPR was undertaken to detect the intermediate of hexavalent chromium reduction. In a mixed waste setting, bioremediation often hinges on understanding the sequence in which electron acceptors will be reduced (Middleton et al. 2003). Cr(V), a d1 paramagnetic species, has a distinct EPR spectrum at conventional X-band frequency that consists of a sharp line at g 1.978 (Myers et al. 2000; Ougden et al. 2004). As there are no known three-electron donors in the biological systems, the reduction of Cr(VI) to Cr(III) cannot occur as a single step (Myers et al. 2000). The presence of Cr(V) detected in the EPR data is indicative of Cr(VI) proceeding through an initial single-electron transfer. As the Cr(V) signal did not increase continually throughout the time course, it is possible that Cr(V) is not the terminal product and that most of the Cr(V) is subsequently reduced to Cr(III), the next stable oxidation state. As Cr(IV) is difficult to detect by EPR, it is not known whether this intermediate was generated as a part of the microbial Cr(VI) reduction process. Cr(V) was also detected by EPR spectroscopy in Shewanella putrefaciens MR-1 (Myers et al. 2000).
Several inhibitors of the ETC were used in this study. Inhibitor studies suggest the involvement of multi-component ETC. Rotenone is the respiration inhibitor of nicotinamide adenine dinucleotide (NADH) dehydrogenase (complex I). Antimycin A is known to inhibit the oxidation of both NADH and succinate, thereby blocking complex III. Cyanide is a strong inhibitor of cytochrome oxidase (complex IV). The inhibition of chromate reduction by MCMB-821 by each of these inhibitors suggested the involvement of ETC in the chromate reduction process and also suggested that Cr(VI) was used as the terminal electron acceptor by MCMB-821. Bacteria use oxygen as a terminal electron acceptor under aerobic conditions and oxyanions, such as chromate, under oxygen-limiting conditions. However, chromate reduction under aerobic conditions is not a common occurrence in bacteria. Cr(VI) has a half-reaction reduction potential comparable to molecular oxygen. It would be interesting to investigate whether MCMB-821 is capable of using Cr(VI) as a preferential electron acceptor over oxygen under non-oxygen-limiting conditions.
DNP (an uncoupler) is known to accelerate the respiratory chain-linked electron transport mechanism, analogous to the stimulation of aerobic respiration by an uncoupling agent wherein electron flow towards the terminal electron acceptor, such as molecular oxygen, is enhanced. The observed increase in the chromate reduction efficiency of MCMB-821 in the presence of DNP may, thus, be attributed to the DNP mediated enhanced electron flow. The enhanced chromate bioreduction in the presence of DNP also supports the hypothesis that Cr(VI) may act as a terminal electron acceptor in bacteria, especially in oxygen-limiting conditions. According to the studies conducted in Escherichia coli ATCC 33456, an inhibitory effect was noted in the presence of this uncoupler. However, the researchers attributed it to membrane distortion or to steric interference (Shen and Wang 1993).
The results, thus, obtained have characterized and identified a new chromate-reducing strain, Burkholderia cepacia MCMB-821, from a pristine habitat of alkaline crater lake. This observation has established that chromate-resistant bacteria are prevalent even in natural ecosystems that are not contaminated with chromate. It was evident from the ETC inhibitor studies that MCMB-821 used Cr(VI) as a terminal electron acceptor. This strain possesses potential of reducing chromate under both aerobic and anaerobic conditions over a wide pH range. Thus, MCMB-821 appears to be of immense use in processes of environmental and biotechnological significance.
References
Ackerley DF, Gonzalez CF, Keyhan M, Blake RII, Matin A (2004) Mechanism of chromate reduction by E. coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stresses during chromate reduction. Environ Microbiol 6:851–860
APHA–AWWA–WPCF (1985) Standard methods for the analysis of water and waste water. American Public Health Association, Washington, pp 201–204
Camargo FAO, Bento FM, Okeke BC, Frankenberger WT (2003) Chromate reduction by chromium resistant bacteria isolated from soils contaminated with dichromate. J Environ Qual 32:1228–1233
Chardin B, Dolla A, Chaspoul F, Fardeau ML, Gallice P, Bruschi M (2002) Bioremediation of chromate: thermodynamic analysis of effects of Cr(VI) on sulfate reducing bacteria. Appl Microbiol Biotechnol 60:352–360
Elangovan R, Abhipsa S, Rohit B, Philip L, Chandraraj K (2006) Reduction of Cr(VI) by a Bacillus sp. Biotechnol Lett 28:247–253
Jeyasingh J, Philip L (2005) Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. J Hazard Mater B 118:113–120
Kodam KM, Soojhawon I, Lokhande PD, Gawai KR (2005) Microbial reduction of reactive azo dyes under aerobic conditions. World J Microbiol Biotechnol 21:367–370
Kreig NR, Holt JG (1984) Gram-negative aerobic rods and cocci. In: Kreig NR, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol I. Williams & Wilkins, Baltimore, pp 141–199
Liu YG, Xu WH, Zeng GM, Tang CF, Li CF (2004) Experimental study on Cr(VI) reduction by Pseudomonas aeruginosa. J Environ Sci (China) 16:797–801
Losi ME, Amrhein C, Frankenberger WT (1994) Environmental biochemistry of chromium. Rev Environ Contamin Toxicol 36:91–121
Mclean J, Beveridge TJ (2000) Chromate reduction by a pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084
Middleton S, Latmani R, Mackey M, Elliman M, Tebo B, Criddle C (2003) Co-metabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell associated reduced chromium and inhibits growth. Biotechnol Bioeng 83:627–637
Myers CR, Carstens BP, Antholine WE, Myers JM (2000) Chromium (VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J Appl Microbiol 88:98–106
Nieboer E, Jusys AA (1988) Biologic chemistry of chromium. In: Nriagu JO, Nieboer E (eds) Chromium natural and human environments. Wiley, New York, pp 21–79
Ougden K, Rigby K, Brooke DM (2004) Oxidative activation of the human carcinogen chromate by arsenite: a model for synergistic metal activation leading to oxidative DNA damage. Toxicol In Vitro 18:741–748
Pal A, Paul AK (2004) Aerobic chromate reduction by chromium resistant bacteria isolated from serpentine soil. Microbiol Res 159:347–354
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by E. coli ATCC 33456. Appl Environ Microbiol 59:3771–3777
Thacker U, Madamwar D (2005) Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol 21:891–899
Upreti RK, Shrivastava R, Chaturvedi UC (2004) Gut microflora and toxic metals: chromium as a model. Indian J Med Res 119:49–59
USEPA (1998) Toxicological review of hexavalent chromium. USEPA, Washington, DC, pp 1–64
Wang P, Mori T, Toda K, Ohtake H (1990) Membrane associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172:1670–1672
Wang CH, Huang CP, Saunders PF (2002) Transport of Cr(VI) in soils contaminated with chromate ore processing residue (COPR). Pract Period Hazard Toxic Radioact Waste Manag 6:6–13
Yadav S, Shukla OP, Rai UN (2005) Chromium pollution and bioremediation. Environews Newsletter of ISEB India 11:6–7
Yassi A, Nieboer E (1988) Carcinogenecity of chromium compounds. In: Nriagu JO, Nieboer E (eds) Chromium natural and human environments. Wiley, New York, pp 443–495
Acknowledgment
The authors thank Dr. Srinivas Darbha and L. Satyanarayana, National Chemical Laboratories, Pune, 411008, India, for the EPR analysis. The financial support provided by the University of Pune is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Characteristics of strain MCMB-821 (DOC 50 kb)
Rights and permissions
About this article
Cite this article
Wani, R., Kodam, K.M., Gawai, K.R. et al. Chromate reduction by Burkholderia cepacia MCMB-821,isolated from the pristine habitat of alkaline crater lake. Appl Microbiol Biotechnol 75, 627–632 (2007). https://doi.org/10.1007/s00253-007-0862-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0862-7