Abstract
A novel support has been utilized for immobilization of lipase, which was prepared by amination of silica with ethanolamine followed by cross linking with glutaraldehyde. Lipases from Rhizopus oryzae3562 and Enterobacter aerogenes were immobilized on activated silica gel, where they retained 60 and 50% of respective original activity. The thermal stability of the immobilized lipases was significantly improved in comparison to the free forms while the pH stability remained unchanged. E. aerogenes and R. oryzae3562 lipases retained 75 and 97% of respective initial activity on incubation at 90 °C, whereas both the free forms became inactive at this temperature. The conversion yield of isoamyl acetate was found to be higher with the immobilized fungal (90 vs. 21%) and bacterial lipases (64 vs. 18%) than the respective free forms. Immobilized R. oryzae3562 lipases retained 50% activity for isoamyl acetate synthesis up to ten cycles whereas it was eight cycles for E. aerogenes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases are amongst the most widely used biocatalysts due to their ability to catalyze diverse reactions. It is extensively used for the catalysis of various chemical reactions in organic solvents, which leads to multiple industrial applications viz. food and flavor making, pharmaceuticals, synthesis of carbohydrate esters, amines and amides, biodetergents and recently cosmetics and perfumery [1–3].
As the recovery yield and reusability of free enzymes as industrial catalysts are quite limited, attention has been paid to enzyme immobilization which may offer advantages over free enzymes such as possibility of reuse, continuous operation, better control of reactions, improved stability and adaptability to various engineering designs [4–6]. Among the various supports available for enzyme immobilization, solid supports are most commonly used as it leads to increased operational stability and enables easier separation from the reaction mixture [7, 8]. Lipase has been immobilized by several methods, namely, adsorption, cross-linking and covalent attachment [9–11]. Among the different immobilization techniques, the adsorption is one of the easiest methods with a higher commercial prospective. Moreover, for catalysis of chemical reaction in organic solvent, adsorption on silica is the simplest, inexpensive and trouble-free immobilization technique because enzymes are normally not soluble in organic media [12, 13]. It is also advantageous to use a porous support so that the enzyme is spread on a large surface area [14, 15]. So in the present study we have used the silica gel, a robust and easily available matrix for immobilization of lipase [16]. But it was observed that although the adsorption techniques are easy to perform, the bonding of the enzyme to the matrix is often weak and such biocatalysts generally lack the degree of stabilization achieved by covalent attachment. So in the present investigation silica was first aminated with ethanolamine followed by cross linking with glutaraldehyde to enhance the degree of immobilization and thereby increased the thermostability of immobilized enzyme.
Thermostable lipases have tremendous applications in enzyme catalyzed reactions, as reactions at higher temperatures have resulted in (a) higher conversion rates, (b) lower possibility of microbial growth, (c) higher substrate solubility, and (d) lower viscosity of the reaction medium thereby favoring mass transfer [17, 18]. There are several reports that covalent bonding between enzyme and support [19, 20], encapsulation in the inorganic microcapsules [21] and entrapment in the alginate gels [22] can increase the thermostability of an enzyme.
In present study we immobilized the R. oryzae3562 and E. aerogenes lipases on activated silica where the thermostability was found to improve significantly compared with their respective free forms. The effect of immobilization on other parameters such as pH stability, K m and V max was also studied. Moreover, the efficiency of free and immobilized lipase for isoamyl acetate synthesis and operational stability of immobilized lipase in organic solvent were investigated.
Materials and methods
Chemicals
Silica gel (60–120 mesh), ethanolamine, acetone and glutaraldehyde were purchased from Merck. All other solvents and reagents were AR grade and were also obtained from Merck.
Lipase production
All experiments were carried out using lipases from R. oryzae3562 and E. aerogenes. The optimum lipase production from E. aerogenes was achieved in submerged fermentation which was in line with earlier report [23]. The extracellular lipase production from E. aerogenes was carried out in 250 mL Erlenmeyer flasks each containing 50 mL of a medium composed of peptone (0.5%), yeast extract (0.3%), NaCl (0.25%), MgSO4 (0.05%), and coconut oil (3.0%) at pH 7.0. Medium was sterilized and inoculated with 3.5 mL (4 × 108 cells/mL) of inoculum followed by incubation for 60 h at 34 °C with shaking at 200 rpm. At the end of the incubation period, supernatant from the fermentation media was collected by the centrifugation at 10,000 rpm for 10 min. The supernatant was treated with acetone (1:4, v/v) for 1 h at 4 °C followed by centrifugation at 10,000 rpm for 10 min. The precipitate was dissolved in 50 mM phosphate buffer and lyophilized for use as a crude lipase preparation for subsequent immobilization.
The extracellular lipase production from R. oryzae3562 was maximum under solid state fermentation, because this fermentation condition resembles its natural habitat [24]. Lipase production from R. oryzae3562 was performed in 250 mL Erlenmeyer flask containing 4 g wheat bran supplemented with 6 mL modified Czapek-dox medium (KH2PO4 1.0 g/L, MgSO4·7H2O 0.5 g/L, KCl 0.5 g/L, NaNO3 2.5 g/L, glucose 50 g/L) at pH 5.0 and 0.6 mL coconut oil. Medium was sterilized and inoculated with 1 mL (106 spores/mL) spore suspension followed by incubation at 35 °C and 85% relative humidity. After 5 days, the fermented biomass was soaked in 16 mL of water followed by extraction through cheesecloth. The supernatant was collected by centrifugation at 10,000 rpm for 10 min and it was treated with ammonium sulphate (60% saturation) at 4 °C. The precipitate was dissolved in 50 mM phosphate buffer (pH 8) and dialyzed by keeping the enzyme in dialysis bag (Hi-media, 12 kDa) overnight in 50 mM phosphate buffer (pH 8.0) under constant stirring. The concentrated enzyme was lyophilized and used as crude lipase for subsequent immobilization.
Preparation of activated silica support
Aminated silica gel was used as a support for the immobilization of the enzyme, which was prepared by refluxing 5 g silica gel in 25 mL ethanolamine. After 3 h silica gel was washed thrice with 60 mL acetone and air dried. This silica gel was then activated using 25 mL 4% (w/v) glutaraldehyde in 50 mM phosphate buffer (pH 8.0) with gentle agitation at 4 °C for 2 h. The activated silica gel was first washed with 50 mM phosphate buffer, pH 8.0 and then with 50 mM phosphate buffer pH 6.5, to make it glutaraldehyde free.
Immobilization of lipases
About 25 mL (30 U/mL) of crude lipases in 50 mM phosphate buffer (pH 8.0) was mixed with 0.1 mL of Tween 80 and stirred for 5 min followed by the addition of 5 g of activated silica gel. Hundred milliliter of chilled acetone was added and the mixture was stirred for 30 min at 4 °C. Silica immobilized lipase was filtered, washed with 25 mL of chilled acetone and lyophilized. The immobilized catalyst was stored at −4 °C. The final activity of immobilized R. oryzae 3562 and E. aerogenes lipases were 90 U/g of support and 75 U/g of support, respectively.
Enzyme assay
Lipase assay was performed spectrophotometrically using p-nitro phenyl palmitate (p-NPP) as the substrate [25]. p-Nitro phenol was liberated from p-nitro phenyl palmitate by lipase-mediated hydrolysis. One unit (U) of lipase was defined as the amount of enzyme that liberates 1 μmol of p-nitro phenol per min under the assay conditions.
Protein determination
The protein concentrations were estimated by Lowry’s method with bovine serum albumin as standard [26]. The amount of protein coupled on silica gel was determined by the difference between the initial amounts of protein introduced in immobilization reaction and the remaining protein in supernatant.
Effect of temperature
The effect of temperature on the free and immobilized lipase activity was observed for p-NPP hydrolysis. The hydrolysis of p-NPP was investigated at various temperatures (10–60 °C), where the p-NPP solution was pre-incubated to reach the desired temperature before the addition of lipase.
Activation energy
The activation energy (Ea) represents the difference in energy between the reactants and the transition state (or activated complex) of a given reaction. Ea was determined from the Arrhenius law: ln(V) = lnA−Ea/(RT) where, A was a constant for particular reaction, V was relative enzyme activity, temperature (T) was in kelvin (K), gas constant (R = 1.987) in cal K−1 mol−1 and (Ea) in Kcal mol−1 [27]. For the determination of Ea, relative enzyme activity (V) was investigated at various temperatures from 283 to 323 K and the Ea was calculated from the slope (−Ea/R) obtained from the linear plot of lnV against 1/T.
pH stability and thermal stability
The effect of pH on the free or immobilized enzymes was examined after pre-incubating the enzyme samples at 30 °C for 60 min at pH 4–11 [50 mM sodium acetate buffer (pH 4, 5), 50 mM potassium phosphate buffer (pH 6, 7), 50 mM Tris–HCl buffer (pH 8, 9), and 50 mM glycine–NaOH buffer (pH 10, 11)]. Then the residual activity was assayed under the standard conditions. The thermostability was investigated by incubating the free and immobilized enzymes at various temperatures (30–90 °C) for 60 min and the residual activity was assayed under the standard conditions. The enzyme activity of the not incubated lipase was taken as 100%.
Kinetics studies
The kinetic constants (K m and V max in Michaelis–Menton equation) for p-NPP hydrolysis by the free and immobilized lipase were determined by examining enzyme activity (V) with increasing concentrations (0.2–1.2 mM) of the substrate ([S]). The Lineweaver–Burk plot of 1/V versus 1/[S] yields a straight line with an intercept on the y-axis of 1/V max and slope K m/V max.
Synthesis of isoamyl acetate by free and immobilized lipases
Transesterification reaction was carried out in screw-capped vials containing 0.1 M of isoamyl alcohol and 0.2 M of vinyl acetate in 10 mL of hexane; reaction was initiated by addition of 80 U of immobilized enzyme. The vials were placed in an orbital shaker (200 rpm) at 40 °C for 24 h along with the respective controls (reaction mixtures without lipase). Samples were taken out at regular intervals, dried over 3 Å molecular sieves and analyzed by gas chromatography.
Quantitative analysis of reaction products
The reaction product isoamyl acetate formed in organic medium was analyzed by injecting the aliquots of the reaction mixture in a gas chromatograph (Agilent GC-6820) equipped with HP-5 capillary column (0.25 μm × 0.25 mm × 30 m) and FID detector. The column temperature was raised from 50 to100 °C and maintained at this temperature for 5 min. The temperatures of the injector and detector were set at 200 and 250 °C, respectively.
Operational stability
Operational stability of immobilized lipases was also studied for isoamyl acetate synthesis. After the completion of reaction, the immobilized lipases were collected by centrifugation at 5,000 rpm for 10 min and washed with hexane in order to remove the reactants adsorbed on matrix. Then the immobilized lipases were resuspended in the same volume of freshly prepared reaction mixture to start a new run and the supernatant was assayed for lipase activity.
Statistical analysis
The results were reported as the mean ± standard deviation and statistical significance was determined by one-way ANOVA (Windows Excel program) at p < 0.05.
Results and discussion
Immobilization of enzyme
Immobilization of R. oryzae3562 and E. aerogenes lipases were carried out on activated silica, where the immobilization yield of lipases on silica were estimated by calculating the specific activities of respective lipases before and after binding to the matrix. Both the R. oryzae3562 and E. aerogenes lipase showed high immobilization yield, i.e., 60 and 50%, respectively on cross linked aminated silica. The present report with 60% immobilization yield is almost ten times higher than that reported by Ghamgui et al. 2004 where lipase from R. oryzae showed only 6.2% of total initial activity when adsorbed on unactivated silica [28]. Although only 50% immobilization yield was achieved with E. aerogenes lipases, which is less than the earlier report of Nawani et al. 2006 but the present study of lipase immobilization on cross linked silica led to improved thermostability unlike the earlier report [29]. It was observed that the activated silica enhances the immobilization yield and thermostability of lipase, which may be due to the strong interaction between enzyme and the matrix. The strong interaction can be further attributed to the formation of Schiff’s base between free aldehyde groups of glutaraldehyde treated silica gel and the side-chain amino groups of the enzyme [16].
Effect of temperature on lipase activity
The immobilization of enzymes by multiple point binding resulted in an increase of enzyme rigidity, which is commonly reflected by increase in stability towards denaturation [30]. In the present study the behavior of free as well as immobilized enzyme was compared for both fungal and bacterial lipase. It has been observed that the fungal and the bacterial free lipases had higher activity than the immobilized enzymes up to 20 and 30 °C, respectively, after that the immobilized lipases were more stable and showed a slight increase in activity (Figs. 1, 2). The activation energy of the free and immobilized lipases obtained from Arrhenius plots were 2.03 and 2.3 Kcal/mol, respectively, for R. oryzae3562 lipase (Fig. 3) while 1.74 and 2.0 Kcal/mol, respectively, for E. aerogenes lipase (Fig. 4). The higher values of activation energy obtained for the immobilized lipases indicated that the applied immobilization procedure introduced changes in the structure of the enzyme molecule, which impeded the enzyme catalyzed reaction [31]. These results are in agreement with the studies reported by Gottschalk and Jaenicke (1991) where the activation energy of free α-amylase was lower than the immobilized form [30].
Thermostability of free and immobilized lipases
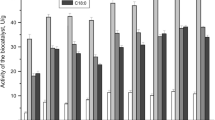
The thermostability of an enzyme is a very important parameter especially in exploring the potential industrial applications. The thermostability of immobilized enzymes might be drastically increased on attachment to a complementary surface of a relatively rigid support [32]. Lipases immobilized on cross linked matrix withstand the adverse effect of higher temperatures, thus improving the thermostability of lipases [29]. The immobilized and free lipases were tested for temperature tolerance over a range of 30–90 °C and a considerable increase in thermostability of immobilized lipases was observed (Fig. 5). In the present study it has been seen that for immobilized lipases of E. aerogenes and R. oryzae3562, there was no activity loss up to a temperature of 60 and 80 °C, respectively, however with the respective free enzymes the stability was only up to 50 and 40 °C. At 90 °C, immobilized bacterial and fungal lipases retained about 75 and 97% of their respective original activity, whereas fungal and bacterial free lipases became inactive at this temperature. The observed thermostability of immobilized lipases was found to be improved than the previous reports. Mucor javanicus lipase immobilized on silica nanoparticles retained 80% of its initial activity at 60 °C after 1 h of incubation [7]. At 60 °C, Candida rugosa lipase immobilized on dry and wet chitosan beads retained an activity of about 45 and 60%, respectively [33]. Lipase from Bacillus sp. when immobilized on HP-20 beads retains approximately 60% of activity at 80 °C after incubation for 1 h [29]. Thermostability depends on the number of bonds acting between the enzyme and the support to prevent the unfolding of the enzyme at increased temperature [34]. So from the present study it can be concluded that a considerable improvement in the thermostability of enzyme have been noticed for both fungal and bacterial system when compared with the other immobilized lipases reported by other researchers. In most cases, including the present study, it has not been possible to clearly elucidate the nature of the interaction between the enzyme and the support. This would require a more systematic investigation with the aid of protein engineering [35].
Effect of pH on lipase activity
Immobilization of enzyme may attribute to the conformational changes of enzyme resulting in a variation of optimum pH. The effect of pH on the lipase activity was studied and it was observed that free as well as immobilized lipases remained stable in the pH range from 4 to 11. This indicated that no conformational change affecting the pH stability of lipase had occurred during immobilization. The results are indicated in Figs. 6 and 7. Similar results on pH stability were reported previously [29, 36].
Kinetic parameters of free and immobilized lipases
The kinetic constants V max and K m were calculated from the double reciprocal plots shown in Figs. 8 and 9. The K m values of bacterial and fungal free lipases (1.41 and 0.3 mM, respectively) were found to be higher than that of the immobilized lipases (0.3 and 0.25 mM, respectively), so immobilization had increased the affinity of enzyme for the substrate. These results were in accordance with those obtained previously [37]. The V max values for both immobilized bacterial and fungal lipases (2.38 U/mg and 0.8 U/mg, respectively) were found to decrease slightly compared to their respective free lipases (2.65 U/mg and 0.85 U/mg, respectively). In general, V max values of enzymes exhibit a decrease on immobilization; which is in accordance with the other similar results [38, 39].
Efficiency of free and immobilized lipase in synthesis reaction
Synthesis of isoamyl acetate by free and immobilized lipases
The capacity of the immobilized and the free lipases to catalyze isoamyl acetate synthesis in n-hexane was investigated. Isoamyl acetate is a short chain ester widely used in food and cosmetic industry due to its pleasant banana flavour [40, 41]. As shown in Fig. 10, a higher percentage conversion of 90% was obtained with the immobilized R. oryzae3562 lipase; however, the conversion percentage does not exceed 21% using the free lipase. Similar results were obtained with E. aerogenes lipase where conversion yields by immobilized and free lipases were 64 and 18%, respectively (Fig. 11). These outcomes were found to be improved than those reported previously [42]. Therefore, the immobilized lipase is an attractive biocatalyst to carry out the synthesis in organic solvents.
Synthesis of isoamyl acetate in hexane by the free and the immobilized E. aerogenes lipase. Reaction conditions were 80 U of lipase, a vinyl acetate/isoamyl alcohol molar ratio of 2 at 40 °C and stirred at 200 rpm. Data are represented as mean ± standard deviation of three replications. The values in the figure denoted by same symbol differ significantly at p < 0.05
Synthesis of isoamyl acetate in hexane by the free and the immobilized R. oryzae3562 lipase. Reaction conditions were 80 U of lipase, a vinyl acetate/isoamyl alcohol molar ratio of 2 at 40 °C and stirred at 200 rpm. Data are represented as mean ± standard deviation of three replications. The values in the figure denoted by same symbol differ significantly at p < 0.05
Operational stability of immobilized lipases
Being an industrial enzyme, repetitive use of the immobilized enzyme is very important as far as the economy of the process is concerned. The operational stability and multiple uses of immobilized lipase can make the process more viable over its free counterpart. Both the bacterial as well as fugal immobilized lipases ensure significant reuse capacity in an organic media; this may attribute to the less leakage of immobilized enzyme in organic solvents. The immobilized R. oryzae3562 and E. aerogenes could be reused up to five and four cycles, respectively, with almost 90% retention of its original activity (Fig. 12). While fungal and bacterial lipase retained 50% of activity after ten and eight cycles, respectively, which is found to be improved when compared with the other reports [43, 44]. While, Candida rugosa lipase immobilized on chitosan beads by the binary method retained 74% residual activity after ten reuses [45].
Conclusion
Immobilization of lipases of R. oryzae3562 and E. aerogenes has been attempted successfully on silica activated with ethanolamine followed by cross linking with glutaraldehyde. Enzyme carrier has been prepared by activation of inexpensive and easily available silica gel. Immobilization has been carried out using adsorption technique that causes less damage to the catalytic activity of the enzyme. In the present investigation it has been seen that the immobilized lipases exhibited quite improved tolerance against thermal denaturation than free forms. The utilization of thermostable enzymes in industrial processes allows reactions to be performed at higher temperature which results in completion of reaction in shorter times. So there is increase demand for thermostable enzymes to replace the thermolabile enzymes in order to improve the overall economy of the process. The immobilized lipases also showed enhanced activities for isoamyl acetate synthesis than free forms, which undoubtedly explain the rationale for using immobilized lipase in organic synthesis. Furthermore, the immobilized lipase also exhibited quite good operational stability for esterification reaction in hexane. Altogether, these results confirmed that the activated silica is a potential support in the enzyme immobilization technology especially for catalyzing reactions in organic media.
References
Rizzi M, Stylos P, Rieck A, Reuss MA (1992) A kinetic study of immobilized lipase catalysing the synthesis of isoamyl acetate by transesterification in n-hexane. Enzyme Microb Technol 14:709–714
Vulfson EN (1994) Lipases––their structure. In: Woolley P, Peterson SB (eds) Biochemistry and Applications. Cambridge University Press, Cambridge, pp 271–288
Welsh FW, Murray WD, Williams RE (1989) Microbiological and enzymatic production of flavor and fragrance chemicals. Crit Rev Biotechnol 9:105–169
Cao L, Langen LV, Sheldon RA (2003) Immobilized enzymes: carrier-bound or carrier free? Curr Opin Biotechnol 14:387–394
Khare SK, Nakajima M (2000) Immobilization of Rhizopus japonicus lipase on celite and its application for enrichment of docosahexaenoic acid in soybean oil. Food Chem 68:153–157
Bagi K, Simon LM, Szajani B (1997) Immobilization and characterization of porcine pancreas lipase. Enzyme Microb Technol 20:531–535
Kim MIl, Ham HO, Oh S-D, Park HG, Chang HN, Choi S-H (2006) Immobilization of Mucor javanicus lipase on effectively functionalized silica nanoparticles. J Mol Catal, B Enzym 39:62–68
Hwang S, Lee K-T, Park J-W, Min B-R, Haam S, Ahn I-S, Jung J-K (2004) Stability analysis of Bacillus stearothermophilus L1 lipase immobilized on surface-modified silica gels. Biochem Eng J 17:85–90
Balcão VM, Paiva AL, Malcata FX (1996) Bioreactors with immobilized lipases: state of the art. Enzyme Microb Technol 18:392–416
Bryjak J, Bachmann K, Pawlow B, Maliszewska I, Trochimczuk A, Kolarz BN (1997) Immobilization of lipase on various acrylic copolymers. Chem Eng J 65:249–256
Kilinç A, Önal S, Telefoncu A (2002) Chemical attachment of porcine pancreatic lipase to crosslinked poly(vinyl alcohol) by means of adipoyldichloride. Process Biochem 38:641–647
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246
Persson M, Mladenoska I, Wehtje E, Adlercreutz P (2002) Preparation of lipases for use in organic solvents. Enzyme Microb Technol 31:833–841
Zhao XS, Bao XY, Guo W, Lee FY (2006) Immobilizing catalysts on porous materials. Materialstoday 9:32–39
Novak Z, Habulin M, Krmelj V, Knez Ž (2003) Silica aerogels as supports for lipase catalyzed esterifications at sub- and supercritical conditions. J Supercrit Fluids 27:169–178
Rao MN, Kembhavi AA, Pant A (2000) Immobilization of endo-polygalacturonase from Aspergillus ustus on silica gel. Biotechnol Lett 22:1557–1559
Bruins ME, Janssen AEM, Boom RM (2001) Thermoenzymes and their applications. Appl Biochem Biotechnol 90:155–186
Gupta MN (1991) Thermostabilization of proteins. Biotechnol Appl Biochem 14:1–11
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb Technol 26:509–515
Petzelbauer I, Kuhn B, Splechtna B, Kulbe KD, Nidetzky B (2002) Development of and ultrahigh temperature process for the enzymatic hydrolysis of lactose. IV. Immobilization of two thermostable β-glycosidases and optimization of a packed-bed reactor for lactose conversion. Biotechnol Bioeng 77:619–631
Matsumoto M, Kondo K (2001) Enhanced thermostability of α-chymotrypsin enclosed in inorganic microcapsules. J Biosci Bioeng 92:197–199
Sharma S, Gupta MN (2001) Alginate as a macroaffinity ligand and an additive for enhanced activity and thermostability of lipases. Biotechnol Appl Biochem 33:161–165
Cheetham PSJ (1995) The applications of enzymes in industry. In: Wiseman A (eds) Handbook of enzyme biotechnology. Ellis Horwood, UK, pp 419–522
Hölker U, Höfer M , Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Kordel M, Hofmann B, Schomburg D, Schmid RD (1991) Ex-tracellular lipase of Pseudomonas sp. strain ATCC-21808, purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol 73:483–484
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Esawy MA, Combet-Blanc Y (2006) Immobilization of Bacillus licheniformis 5A1 milk-clotting enzyme and characterization of its enzyme properties. World J Microbiol Biotechnol 22:197–200
Ghamgui H, Karra-Chaâbouni M, Gargouri Y (2004) 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: a comparative study between n-hexane and solvent-free system. Enzyme Microb Technol 35:355–363
Nawani N, Singh R, Kaur J (2006) Immobilization and stability studies of a lipase from thermophilic Bacillus sp: the effect of process parameters on immobilization of enzyme. Electron J Biotechnol 9:DOI: 10.2225/vol9-issue5-fulltext-9
Gottschalk N, Jaenicke R (1991) Authenicity and reconstitution of immobilised enzymes characterisation denaturation renaturation of glucoamylase. Biotechnol Appl Biochem 14:324–335
Krajewska B, Leszko M, Zaboraska W (1990) Urease immobilised on chitosan membrane: preparation and properties. J Chem Tech Biotechnol 48:337–350
Martinek K, Kilbanov AM, Goldmacher VS, Berezin IV (1977) The principles of enzyme stabilization. Biochim Biophys Acta 485:1–12
Chiou S-H, Wu W-T (2004) Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 25:197–204
Klibanov AM (1979) Enzyme stabilization by immobilization. Anal Biochem 93:1–25
Matsumoto M, Ohashi K (2003) Effect of immobilization on thermostability of lipase from Candida rugosa. Biochem Eng J 14:75–77
He F, Zhuo RX, Liu LJ, Jin DB, Feng J, Wang XL (2001) Immobilized lipase on porous silica beads: preparation and application for enzymatic ring-opening polymerization of cyclic phosphate. React Funct Polym 47:153–158
Iborra JL, Castellar MR, Cánovas M, Manjón A (1992) Picrocrocin hydrolysis by immobilized fi-glucosidase. Biotechnol Lett 14:475–480
Ye P, Xua Z-K, Che A-F, Wub J, Seta P (2005) Chitosan-tethered poly (acrylonitrile-co-maleic acid) hollow fiber membrane for lipase immobilization. Biomaterials 26:6394–6403
Li S, Hu J, Liu B (2004) Use of chemically modified PMMA microspheres for enzyme immobilization. BioSystems 77:25–32
Krishna SH, Divakar S, Prapulla SG, Karanth NG (2001) Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J Biotechnol 87:193–201
Romeroa MD, Calvoa L, Albaa C, Daneshfarb A, Ghaziaskarb HS (2005) Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzyme Microb Technol 37:42–48
Melo LLMM, Pastore GM, Macedo GA (2005) Optimized synthesis of citronellyl flavour esters using free and immobilized lipase from Rhizopus sp. Process Biochem 40:3181–3185
Carta G, Gainer JL, Benton AH (1991) Enzymatic synthesis of esters using an immobilized lipase. Biotechnol Bioeng 37:1004–1009
Desai PD, Dave AM, Devi S (2006) Alcoholysis of salicornia oil using free and covalently bound lipase onto chitosan beads. Food Chem 95:193–199
Hung T-C, Giridhar R, Chiou S-H, Wu W-T (2003) Binary immobilization of Candida rugosa lipase on chitosan. J Mol Catal, B Enzym.26:69–78
Acknowledgments
The authors wish to acknowledge CSIR, Government of India and Department of Biotechnology, India for providing research fellowship to Annapurna Kumari and Paramita Mahapatra, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumari, A., Mahapatra, P., Kumar, G.V. et al. Comparative study of thermostabilty and ester synthesis ability of free and immobilized lipases on cross linked silica gel. Bioprocess Biosyst Eng 31, 291–298 (2008). https://doi.org/10.1007/s00449-007-0160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0160-x