Abstract
The performance of a biological Fe2+ oxidizing fluidized bed reactor (FBR) was modeled by a popular neural network-back-propagation algorithm over a period of 220 days at 37 °C under different operational conditions. A method is proposed for modeling Fe3+ production in FBR and thereby managing the regeneration of Fe3+ for heap leaching application, based on an artificial neural network-back-propagation algorithm. Depending on output value, relevant control strategies and actions are activated, and Fe3+ production in FBR was considered as a critical output parameter. The modeling of effluent Fe3+ concentration was very successful, and an excellent match was obtained between the measured and the predicted concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most important methods used in biohydrometallurgy involve heap, bioleaching vat and tank biooxidation. Heap leaching is economically the most important of these methods. The bioleaching of sulfide minerals occurs in an acidic medium that often contains a considerable concentration of Fe3+. During the bioleaching applications, the sulfidic mineral is chemically oxidized by microbially generated Fe3+ and the resulting Fe2+ iron is biologically regenerated to Fe3+. However, temperature and pH of heap leaching solution can vary widely over time. Further, as these parameter values increase, Fe3+ precipitation increases. Hence, Fe3+ concentration within the recycled leaching solution to the heap decreases and accordingly the leaching efficiency reduces. Therefore, it is very important to predict Fe3+ concentration recycled to heap by a comprehensive model for the design, monitoring, and management of heap bioleaching operations. As an alternative to physical models, artificial neural networks (ANNs) are a valuable forecast tool. So far, there are several applications of ANN models in the engineering area. For example, Strike et al. [1] used ANN to model H2S and NH3 components of biogas from anaerobic digestion; Clair and Ehrman [2] used ANN to simulate the effect of climate change on discharge and the export of dissolved organic carbon and nitrogen from river basin; Nunnari et al. [3] applied ANN to model air pollution; Cinar [4] used ANN to analyze the system behavior and to determine operational problems of a full-scale activated sludge wastewater treatment plant; Cinar et al. [5] used ANN to evaluate the performance of a membrane bioreactor; Karaca and Özkaya [6] used ANN to predict the leachate quantity from a full-scale municipal solid waste landfill; Ozkaya et al. [7] used ANN to estimate methane fraction in biogas from field-scale landfill; Holubar et al. [8] used ANN to predict biogas production and composition; Sahinkaya et al. [9] used ANN to determine the performance of high rate sulfidogenic fluidized bed reactor treating acidic metal containing wastewater, neural network as a tool. There are also other similar engineering applications of ANN.
In order to effectively extract valuable metals from the minerals, the proper operation and control of bioleaching applications have become very important. According to current understanding the dissolution of metals occurs purely chemically with the help of Fe3+ and H+ ions, which act as oxidizing agents [10]. Better control of bioleaching may be achieved by the use of a robust model to predict certain key parameters based on past observations. Models based on ANNs may be successfully used in bioleaching applications and very effective at capturing the nonlinear relationships existing between variables (multi-input/output) in complex system like bioleaching. This study is aimed at using this ability of artificial neural network for evaluating Fe3+ production in fluidized bed reactor (FBR) and thereby managing heap leaching application. In this study, an artificial neural network based Fe3+ prediction method (ANN-HEAP) using the back-propagation algorithm was proposed to predict effluent Fe3+ concentration of FBR under different operational conditions.
Experimental
Analyses
Total iron was analyzed by atomic absorption spectrophotometer (Perkin Elmer, 1100B). The Fe2+ concentration was determined using the Shimadzu UV 1601 spectrofotometer (Shimadzu, Japan) by the colorimetric ortho-phenantroline method, according to modified 3500-Fe method [11]. Dissolved oxygen (DO) and pH were measured using WTW OXI96, and pH 330i® (Weilheim, Germany) pH meter, and WTW pH-Electrode Sentix41® (Weilheim, Germany), respectively.
The acidophilic iron-oxidizing culture was obtained from a FBR, long-term fed with 7 g Fe2+/L, and nutrient medium containing (g/L): (NH4)2HPO4 (0.35), K2CO3 (0.05), and MgSO4 (0.05) at pH 0.9. The microbial community was monitored by phase contrast microscopy and by using denaturing gradient gel electrophoresis (DGGE) of polymerase chain reaction (PCR) amplified partial 16S rRNA genes as previously described [12]. DGGE analysis was performed with total DNA extracted from the operating FBR carrier at different time intervals to reveal possible changes in bacterial community over time. The sequencing of the purified products was performed at DNA Sequencing Facility, Institute of Biotechnology, Helsinki University, Finland.
Fluidized bed reactor
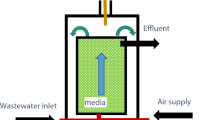
Fluidized bed reactor (FBR) with activated carbon (Kaiser 0.4–1.4 mm) as biomass carrier at 37 °C was used for continuous-flow experiments (Fig. 1). Total and fluidized bed volumes of FBR were 500 and 340 mL, respectively. The recycle flow rate was adjusted to maintain the fluidization ratio at 30%. Air was used for aeration and the aeration system was connected to the recycle flow line of the FBR (Fig. 1). A settling tank with the capacity of 25 L (hydraulic retention time = 2.4 h) was installed on day 50 and used thereafter. The solid retention time of the settling unit was 1 week.
Operational data under different conditions over 220 days (total number of data points were 96) were used in artificial neural network modeling. FBR was operated using three different operation regimes as shown in Table 1.
In order to increase the biomass concentration in the FBR, between days 0 and 40 (Regime-1), the reactor was only fed with 7 g Fe2+/L in a growth medium (Table 1) at pH 0.9. After the day 40, the FBR feed was changed to a simulated heap leaching solution and growth medium. The feed pH of the FBR was gradually increased from 1.5 to 2.5 by decreasing the amount of H2SO4 added to the feed. While the hydraulic retention time (HRT) was kept constant at 5 h, the feed pH was gradually increased from 1.5 to 2.5 (Regime-2). Thereafter, the FBR was operated at HRT between 1.5 and 5 h at pH 2 (Regime-3). Detailed information on the FBR and process variables has been reported by Ozkaya et al. [13].
Fluidized bed reactor performance
The change of feed to the simulated heap leaching solution significantly increased the effluent Fe2+ concentration and inorganic precipitate formation within the FBR. The iron oxidation rate decreased from 3.5 to around 2.0 g Fe2+/L h between days 40 and 50, respectively. A settling tank was installed to the recycle line and used from day 50 onwards, to continuously remove the precipitate from the solution. With the use of settling tank, the Fe2+ concentration decreased from 8,000 to 400 mg/L within 15 days and Fe2+ oxidation was almost complete (98.5%). With the initial simulated solution pH of 1.5, the pH of FBR effluent varied in the range of 1.9–1.6. At feed pH of 1.8 and 2, the effluent pH was about 1.6 and 1.8, respectively. Finally, when pH was increased to 2.5, the effluent pH was around 2. The precipitation of Fe3+ even at pH of 1–1.5 cannot be completely avoided. The incorporation of a gravity settler to the system solved the clogging problems caused by the precipitates of Fe3+ and other inorganic ions. Thereafter, the effect of the loading rate on Fe2+ oxidation was studied using various HRTs (1.5–5 h) for 60 days at pH 2. At loading rates below 10.7 g Fe2+/L h, the Fe2+ conversion was independent of loading rate and remained constant at above 96%. The Fe2+ oxidation rate was not a function of the HRT in this period (days 117–147). At loading rate above 10.7 g Fe2+/L h, the increase in the Fe2+ oxidation rate decreased due to the oxygen mass transfer limitation. During this period, DO sharply decreased from 2.50 to 0.25 mg/L and accordingly effluent Fe2+ concentration increased from 800 to 9,500 mg/L. Due to the oxygen mass transfer limitation, Fe2+ conversion was 55% during this period [13].
The microbial community was monitored microscopically using phase contrast and by using molecular methods. Figure 2 shows the results of PCR–DGGE followed by partial sequencing of 16 S rRNA genes (a) and a phase contrast micrograph (b) of the Fe2+ oxidizing culture. The results show that the FBR operation at different pH values did not affect the biofilm composition and the bacterial community was dominated by Leptospirillum ferriphilum (100% similarity).
After completing the experimental studies, we designed a neural network based FBR effluent Fe3+ prediction method (ANN-HEAP) for evaluating and managing Fe3+ recycle to heap by input parameters selected considering the operational conditions with the help of MATLAB® computer program. A total of six input parameters such as influent and effluent pH, redox potential, HRT, DO, and Fe2+ loading rate were defined, which are essential for accurate modeling of effluent Fe3+ using experimental results.
Artificial neural network based FBR effluent Fe3+ prediction model
The proposed model for modeling Fe3+ recycle to the heap considering Fe3+ precipitation is based on back-propagation (BP) algorithms and was constructed as reported by Karaca and Özkaya [6] (Fig. 3). BP algorithms use input vectors and corresponding target vectors to train an ANN. ANN with a sigmoid and linear output layer are capable of approximating any function with a finite number of discontinuities [14]. The standard BP algorithm is a gradient descent algorithm, in which the network weights are changed along the negative of the gradient of the performance function [15, 16]. There are a number of variations of the basic BP algorithm, which are based on other optimization techniques, such as conjugate gradient and Newton methods. For properly trained BP networks, a new input leads to an output similar to the correct output. This ANN property enables the training of a network on a representative set of input/target pairs and getting good forecasting results.
After back-propagation training, the ANN model predicts Fe3+ percent based on operational conditions of FBR (influent and effluent pH, redox, HRT, DO, and Fe2+ loading rate). The ANN-HEAP model (Fig. 3) has the following steps. (1) For a given collected data from FBR, the best fitting back-propagation algorithm, minimizing the error between ANN output and target value, is selected. (2) The ANN outputs are established, using experimental data. If the percent of Fe3+ recycled to the heap is higher than the threshold value (Fig. 3) relevant actions and warnings are proposed. Fe3+ production percent by FBR may be used as threshold value (<70%).
This ANN has k input and one output parameters, which are essential for accurate modeling of the Fe3+ percent. The input parameters, number of neurons at hidden layer and output layer, should be determined according to currently gathered data.
Selection of back-propagation algorithm
Thirteen BP algorithms were compared to select the best fitting BP algorithm of the gathered data. For all algorithms, a two-layer network with a tan-sigmoid transfer function at the hidden layer and a linear transfer function at the output layer were used.
The learning rate parameter may also play an important role in the convergence of the network, depending on application and network architecture. The learning rate can be used to increase the chance of preventing the training process being trapped in a local minimum instead of a global minimum [17]. The larger the learning rate, the bigger the step. If the learning rate is made too large, the algorithm becomes unstable. If the learning rate is set too small, the algorithm takes a long time to converge. In addition, the momentum allows a network to respond, not only to the local gradient, but also to recent trends in the error surface. Without momentum, a network may get stuck in a shallow local minimum [14]. In this study, the learning rate and the momentum constant were 0.1 and 0.9, respectively. The training results are given in Table 2.
The BP network is one of the several networks that is widely used for predicting the output and is successfully applied to a wide range of problems [6, 7, 9]. The best BP algorithm for the present application, with minimum training error (0.0489), is the Levenberg–Marquardt algorithm (Table 2).
The training stopped after ten iterations because the validation error started to increase (Fig. 4). This result is reasonable, as the test set error and the validation set error have similar characteristics, and it does not appear that any significant change over fitting has occurred.
Optimization of neural network structure for ANN-HEAP model
A total of six parameters were defined, which are essential for accurate modeling of Fe3+ percent recycled to heap using experimental data from FBR combined with settling tank. Input parameters are pH (feed and effluent of FBR), redox potential, DO, HRT, and Fe2+ loading rates which are key parameters indicating FBR performance (Fig. 5). The data were divided into P and T matrices. P matrix contains the input parameters and T matrix contains the target of the ANN. The data were divided into training, validation, and test subsets. One-fourth of the data were taken for the validation set, one-fourth for the set and one half for training.
Optimization of a neural network is an important task of neural network based studies and there are some methods applied [18]. In our study, neuron numbers and relevant performance of the Levenberg–Marquardt algorithm were evaluated. Increasing neuron numbers to more than 10 (Fig. 4) caused an unrealistic result, since the test set and validation set errors have dissimilar characteristics, and a significant change over fitting occurred. Therefore, the optimal neuron number for Levenberg–Marquardt algorithm is 10. The optimal neural network structure for the ANN-HEAP method is given in Fig. 5: a two-layer network, with a tan-sigmoid transfer function at the hidden layer with ten neurons and a linear transfer function at the output layer.
A regression analysis of the network response between the output and the corresponding target was performed. For the output, one regression was determined (Fig. 6) taking into account the nonlinear dependence of the data, the output seemed to track the targets reasonably well. The R value is 0.901.
The performance of the ANN-HEAP model is visualized for Fe3+ concentration in Fig. 7. There is a very good agreement in the trends between predicted and measured data.
Figure 7 has three critical points for FBR performance under different operational conditions. Firstly, Fe3+ concentration significantly decreases due to the change of feed to simulated heap leaching solution associated with inorganic precipitate formation within the FBR. Secondly, FBR performance decreases due to the high pH. Thirdly, reactor performance decreases due to the oxygen mass transfer limitation. All critical points and regular Fe3+ level were successfully predicted by artificial neural network-back-propagation algorithm (ANN-HEAP).
The ANN-HEAP model has the advantage of predicting (not measuring) Fe3+ percent before leaching efficiency becomes reduced, giving sufficient time to take appropriate action. If the amount is predicted at levels lower than the threshold for a heap, appropriate measures are proposed. Suitable warnings are introduced, such as presenting these values on a display in the control center and sending information to higher authorities. In our case study, the sequence is summarized as follows.
The threshold value is taken as 70%. If the Fe3+ percent recycled to heap falls below this threshold, an initial stage of actions and warnings are launched. A flashing control format of rapid actions could: (1) send information to higher authorities, (2) send information to FBR operator, (3) control the FBR performance and measure pH, redox, dissolved oxygen, etc., and (4) consider Fe3+ precipitation in settling tank.
At the same time, other actions may be initiated, such as a special warning to the relevant authorities, example, “Fe3+ percent of concern”. The ANN-HEAP then checks the FBR as a “special decision” (Fig. 3). If there is a problem, special actions and warnings are taken. After the Fe3+ returns to normal levels, regular treatment and control strategies are resumed. Fe3+ levels may be announced on boards in the control center as “suitable Fe3+ level”.
Conclusions
This study demonstrates that ANNs provide a robust tool for predicting the Fe3+ concentration of FBR combined with a settling tank to recycle line to remove the inorganic precipitate from the solution. The effective and robust control and management system was developed by considering predicted Fe3+ concentration for indirect heap leaching applications even though such applications involve highly complex physical and biochemical mechanisms. This study proposes a neural network based model (ANN-HEAP) for Fe3+ concentration evaluation and control. The proposed model can reliably predict effluent Fe3+ concentration and, consequently offer the appropriate warning signal and relevant actions to be taken by the authorities or management of the facility to effectively extract valuable metals from sulfidic minerals in indirect heap leaching application. The advantages of ANN-HEAP model are precise and effective prediction of Fe3+ recycled to heap in an indirect leaching application, and an effective, powerful control and management system.
References
Strike DPBTB, Domnanovich AM, Zani L, Braun R, Holubar P (2005) Prediction of trace compounds in biogas from anaerobic digestion using the MATLAB neural network toolbox. Environ Model Softw 20:803–810
Clair TA, Ehrman JM (1996) Variations in discharge and dissolved organic carbon and nitrogen export from terrestrial basins with changes in climate: a neural network approach. Limnol Oceanogr 41(5):921–927
Nunnari G, Dorling S, Schlink U, Cawley G, Foxall R, Chatterton T (2004) Modelling SO2 concentration at a point with statistical approaches. Environ Model Softw 19(10):887–905
Cinar Ö (2005) New tool for evaluation of performance of wastewater treatment plant: artificial neural network. Process Biochem 40:2980–2984
Cinar Ö, Hasar H, Kinaci C (2006) Modeling of submerged membrane bioreactor treating cheese whey wastewater by artificial neural network. J Biotechnol 123:204–209
Karaca F, Özkaya B (2006) NN-LEAP: a neural network-based model for controlling leachate flow-rate in a municipal solid waste landfill site. Environ Model Softw 21(8):1190–1197
Ozkaya B, Demir A, Bilgili MS (2007) Neural network prediction model for the methane fraction in biogas from field-scale landfill bioreactors. Environ Model Softw 22(6):815–822
Holubar P, Zani L, Hager M, Fröschl W, Radak Z, Braun R (2002) Advanced controlling of anaerobic digestion by means of hierarchical neural networks. Water Res 36:2582–2588
Sahinkaya E, Ozkaya B, Kaksonen AH, Puhakka JA (2007) Neural network prediction of thermophilic (65 °C) sulfidogenic fluidized-bed reactor performance for the treatment of metal-containing wastewater. Biotechnol Bioeng 97(4):780–787
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy 84(1–2):81–108
Anonymous. (1992) American public health association. In: Greenberg AE, Clesceri LS, Eaton AD (eds) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC
Ozkaya B, Sahinkaya E, Nurmi P, Kaksonen AH, Puhakka JA (2007) Kinetics of iron oxidation by Leptospirillum ferriphilum dominated culture at pH below one. Biotechnol Bioeng 97(5):1121–1127
Ozkaya B, Sahinkaya E, Nurmi P, Kaksonen AH, Puhakka JA (2007) Iron oxidation and precipitation in a simulated heap leaching solution in a leptospirillum ferriphilum dominated biofilm reactor. Hydrometallurgy 88(1–4):67–74
Hagan MT, Demuth HB, Beale MH (1996) Neural network design. PWS Publishing, Boston, MA
Nguyen D, Widrow B (1990) Improving the learning speed of 2-layer neural networks by choosing initial values of the adaptive weights. In: Proceedings of the international joint conference on neural networks, pp 321–326
Abdi H, Valentin D, Edelman B, O’Toole AJ (1996) A Widrow–Hoff learning rule for a generalization of the linear auto-associator. J Math Psychol 40(2):175–182
Hamed MM, Khalafallah MG, Hassanien EA (2004) Prediction of wastewater treatment plant performance using artificial neural networks. Environ Model Softw 19(10):919–928
Almasri MN, Kaluarachchi JJ (2005) Modular neural networks to predict the nitrate distribution in groun water using the onground nitrogen loading and recharge data. Environ Model Softw 20(7):851–871
Acknowledgments
This research was funded by the Talvivaara Mining Company Ltd. Authors Bestamin Özkaya and Erkan Sahinkaya would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozkaya, B., Sahinkaya, E., Nurmi, P. et al. Biologically Fe2+ oxidizing fluidized bed reactor performance and controlling of Fe3+ recycle during heap bioleaching: an artificial neural network-based model. Bioprocess Biosyst Eng 31, 111–117 (2008). https://doi.org/10.1007/s00449-007-0153-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0153-9