Abstract
Jack bean urease (urea aminohydrolase, E.C. 3.5.1.5) was entrapped into chitosan–alginate polyelectrolyte complexes (C-A PEC) and poly(acrylamide-co-acrylic acid)/κ-carrageenan (P(AAm-co-AA)/carrageenan) hydrogels for the potential use in immobilization of urease, not previously reported. The effects of pH, temperature, storage stability, reuse number, and thermal stability on the free and immobilized urease were examined. For the free and immobilized urease into C-A PEC and P(AAm-co-AA)/carrageenan, the optimum pH was found to be 7.5 and 8, respectively. The optimum temperature of the free and immobilized enzymes was also observed to be 55 and 60 °C, respectively. Michaelis–Menten constant (K m) values for both immobilized urease were also observed smaller than free enzyme. The storage stability values of immobilized enzyme systems were observed as 48 and 70%, respectively, after 70 days. In addition to this, it was observed that, after 20th use in 5 days, the retained activities for immobilized enzyme into C-A PEC and P(AAm-co-AA)/carrageenan matrixes were found as 55 and 89%, respectively. Thermal stability of the free urease was also increased by a result of immobilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzyme urease (E.C. 3.5.1.5) is a highly specific enzyme and catalyzes the hydrolysis of urea to ammonium and carbon dioxide [1]. It has been immobilized for various analytical and biomedical purposes. One of the main applications of immobilized urease is the direct removal of urea from blood for detoxification [2] or in the dialysis regeneration systems of artificial kidney machines [3]. Other applications of immobilized urease will be in a bioreactor for the conversion of urea present in fertilizer wastewater effluents to ammonia and carbon dioxide [4] or in the food industry for the removal of urea from beverages and foods [5, 6].
Recently, there has been an increasing interest in use of naturally occurring polymers as support material for immobilization of proteins. Chitosan and alginate are both polysaccharides derived from marine biopolymers. Chitosan is a copolymer of glucosamine and N-acetylglucosamine obtained by deacetylation of chitin. Alginate is an acidic linear polysaccharide composed of two sugars, l-guluronic acid and d-mannuronic acid, in variable proportions. The amino group of chitosan is easily protonated at moderately low pH (< 6.3) to form cationic polyelectrolyte, and the carboxylic acid group of alginate is easily ionized in water to form anionic polyelectrolyte. The polycation and polyanion, carrying electrostatically complementary ionizable groups, can form a stable polyelectrolyte complex. Beads prepared from chitosan–alginate polyelectrolyte complex have gained considerable attention in tissue engineering as scaffold and controlled-delivery systems for macromolecular drugs [7–10].
Interpenetrating polymer networks (IPNs) are mixtures of two cross-linked polymers. Generally, IPNs are formed either by simultaneous parallel reactions according to various mechanisms or by swelling one network in monomers from which the second network can be produced [11]. IPNs are preferred in a number of biotechnological and biomedical applications due to their certain biophysical properties such as ease of fabrication to various geometrical forms, soft and rubbery texture, and unusual stability to biofluids [12].
The objective of the present investigation was to offer a simple method for the preparation of chitosan–alginate polyelectrolyte complex (C-A PEC) and IPNs prepared from poly(acrylamide-co-acrylic acid)/κ-carrageenan (P(AAm-co-AA)/carrageenan) as supports for immobilization of urease. Immobilization of urease by using various materials has been reported by many researchers in the literature [13–19]. As far as we aware the immobilization of urease by these matrixes has not been reported in the literature. These polymer matrixes have certain advantages over other materials such as low cost, ease of enzyme accessibility, hydrophilic character, etc. For this purpose, the Michaelis–Menten kinetic constants (K m and V max), optimum pH and temperature, reuse numbers, storage and thermal stability for the free and immobilized urease were reported.
Experimental
Materials
Jack bean urease (E.C. 3.5.1.5), chitosan, sodium alginate, and urea were purchased from Sigma-Aldrich Chemical Company (St. Louis, USA). Acrylamide (AAm), acrylic acid (AA), ammonium persulphate (APS), N,N,N′,N′-tetramethylethylendiamine (TEMED), N,N′-methylene-bis-acrylamide (BAAm), acetic acid, phosphoric acid, boric acid, and all the other chemicals were obtained from Merck AG (Darmstadt, Germany).
Glacial acetic acid, phosphoric acid (85%, v/v), boric acid, and standardized NaOH were used to prepare Britton–Robinson (B-R) buffers. B-R buffer solution was prepared in such a way that 2.3 mL glacial acetic acid, 2.7 mL phosphoric acid, and 2.4720 g boric acid dissolved by dilution triple-distilled water to 1.0 L; 50 mL portions of this solution were taken and the pH was adjusted between 4.0 and 8.0 by the addition of an appropriate amount of 2.0 M NaOH.
Immobilization of urease into Chitosan–Alginate polyelectrolyte complex and P(AAm-co-AA)/carrageenan hydrogels
Urease was entrapped into C-A PEC and IPNs of P(AAm-co-AA)/carrageenan. In the first part of this study, chitosan and alginate solutions were prepared separately by dissolving chitosan powder (0.5 g) in 50 mL of distilled water containing acetic acid (1.0% by weight) and sodium alginate powder (0.5 g) in 50 mL distilled water at room temperature, respectively. The two solutions were then mixed thoroughly for 6 h. Then, the dissolved chitosan solution was added into the alginate solution and blended with a magnetic stirrer for 2 h. After this process, enzyme urease (6 mL) was added into this solution and stirred thoroughly for 1 h. This homogeny mixture was dropped into 0.27 M CaCl2 solution. As a result of this procedure, urease was entrapped in C-A PEC spherical beads in 2 mm diameters. The immobilized enzymes were washed twice with distilled water. It was stored at 4 °C in buffer solution until use.
In the second part of this study, enzyme urease was entrapped into IPNs of poly(acrylamide-co-acrylic acid)/carrageenan. The immobilization procedure was carried out as following ways. P(AAm-co-AA)/carrageenan hydrogels were synthesized by free-radical cross-linking polymerization of AAm and AA using a small amount of bisacrylamide (BAAm) as the cross-linker. APS and TEMED were used as the redox initiator system. In 20 mL distilled water, AAm (2.85 g), AA (2 mL), κ-carrageenan (1 g), bisacrylamide (0.15 g), APS (10 mg), enzyme solution (6 mL), and TEMED (1 mL) were mixed thoroughly. This mixture was stirred and poured into a flat bottom petri dish. After polymerization, the P(AAm-co-AA)/carrageenan hydrogels were cut into equal size cubes (5 × 5 × 5 mm3). The gel cubes were waited in distilled water for 1 h in the refrigerator. These cubes were washed thoroughly three times with distilled water. Enzyme immobilized hydrogels were stored at 4 °C in buffer solution used in the further experiments.
Enzyme activity assay
Urease activity of both soluble and immobilized enzyme was determined by the Nessler ammonia assay method using a Unicam UV/Vis spectrophotometer UV2. The method is based on the hydrolysis of urea to ammonium [20, 21]. Unless otherwise stated, the reaction mixture contained 1 mL of 0.2 M urea, 1 mL B-R buffer (pH 7.5), an appropriate amount of the enzyme (soluble enzyme, 0.1 mL; C-A PEC beads, 0.5 g; P(AAm-co-AA)/carrageenan, 0.5 g). This mixture was kept in a shaking water bath and the reaction was carried out at 55 °C for 30 min. Then, 1 mL trichloroacetic acid was added into this mixture. For estimating the amount of ammonium, to a suitable aliquot (generally 1 mL) of the reaction mixture, 1 mL solution of Nessler reagent was added. Absorbance at 391 nm was recorded. Activities of enzymes were determined by using the slope of the ammonium calibration curve.
Results and discussion
Effects of pH and temperature on activity
The change in the optimum pH depends on the charge of the enzyme and/or of the matrix. This change is useful in understanding the structure–function relationship of the enzyme and to compare the activity of free and immobilized enzyme as a function of pH. The effect of pH on the activity of the free and the immobilized urease was assayed in the pH range of 5.0–10.0 at 35 °C. The results are presented in Fig. 1. As shown in the figure, for the free enzyme and the immobilized enzymes into C-A PEC and P(AAm-co-AA)/carrageenan, the maximum activities were observed at pH 7.5 and 8.0, respectively. In the cases of C-A PEC and P(AAm-co-AA)/carrageenan immobilized urease, the optimum pH shifted by 0.5 unit toward the alkaline region (Fig. 1). Generally, immobilized enzyme on polycationic supports would result in an acidic shift in the pH optimum [22]. These shifts may be attributed to secondary interactions, such as ionic and polar interactions, hydrogen bonding, etc., between the enzyme and the polymeric matrix [23, 24]. On the other hand, the results suggested that the immobilized ureases possessed better pH stability than the free one.
The effect of temperature on the activity of free and immobilized ureases for urea hydrolysis in the temperature range of 30–70 °C is shown in Fig. 2. As shown in the figure, it was found that the optimum temperature for the free and immobilized enzyme into P(AAm-co-AA)/carrageenan was 55 °C, while it shifted to 60 °C for the immobilized enzymes into C-A PEC. This was due to either the creation of conformational limitation on the enzyme movement as a result of ionic interactions between the enzyme and the supports or a low restriction in the diffusion of the substrate at high temperature. Thus, the immobilized enzymes showed their catalytic activities at a higher reaction temperature.
Storage stability
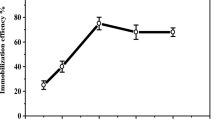
The stability of an enzyme is of significant importance for scheduling its application in a particular reaction. The immobilized and free ureases were stored in B-R buffer solution at 4 °C for various time periods. The residual activity of the enzyme was determined as a function of time using urea as substrate and results are given in Fig. 3. It is observed that the free enzyme looses its 50% activity within 7 days. In the same time frames, immobilized enzymes into C-A PEC and P(AAm-co-AA)/carrageenan retained about 94 and 98% of its original activity. After 20 days, the catalytic activity of the free urease was fully lost. Upon 70 days of storage C-A PEC and P(AAm-co-AA)/carrageenan immobilized enzyme retained about 48 and 70% of its original activity, respectively. The generated multi-point ionic interactions between enzyme and supports should also convey a higher conformational stability to the immobilized enzyme. Thus, C-A PEC and P(AAm-co-AA)/carrageenan supports and the immobilization methods provide higher shelf life for the immobilized forms compared to that of its free form [23, 24].
Kinetic parameters
The effect of the substrate concentration on the kinetics of the reaction catalyzed by free and immobilized ureases was studied using urea as substrate at constant temperature and pH. From the Lineweaver–Burk plot of 1/V versus 1/[S] Michaelis constant (K m) and the maximum reaction velocity (V max) of the free and immobilized enzymes were calculated and results are shown in Fig. 4. The V max value of the free urease (0.0182 mM min−1) was found to be higher than that of the immobilized ureases in the C-A PEC (0.0104 mM min−1) and P(AAm-co-AA)/carrageenan (0.0099 mM min−1). As for, K m values were estimated as 4.5, 3.03, and 3.30 mM for the free, and the immobilized enzymes into C-A PEC and P(AAm-co-AA)/carrageenan, respectively. There was a decrease in K m values for both the immobilized enzymes. Generally, K m values of enzymes demonstrate an increase upon immobilization. However, in this study, it was interesting that the K m values of the immobilized ureases were shown a decrease upon immobilization. These results may be attributed to electrostatic interactions between the substrate and polymeric matrix. Enzyme–substrate complex formation becomes more difficult due to the structure of polymers (porosity and rigid structure) for immobilized enzymes and this also causes a decrease in V max values compare to free enzyme. On the other hand, K m and V max values of the immobilized enzymes were quite close to each other most probably because of similar hydrogel structures of the matrixes.
Thermal stability
Thermal stability experiments were performed with the free and the immobilized ureases in C-A PEC and P(AAm-co-AA)/carrageenan, which were incubated in the absence of substrate at various temperatures. The relative activity as a function of time and temperature is shown in Fig. 5. As seen from the figure, at 65 and 75 °C, both immobilized ureases were inactivated at a much slower rate than those of the free one. At 65 °C, the free and the immobilized urease in C-A PEC and P(AAm-co-AA)/carrageenan retained their activity about to a level of 21, 50, and 60% during a 50 min incubation period, respectively. In addition, at 75 °C, the free and the immobilized ureases retained about 4, 33.4, and 55.5% of their original activities, respectively. The conformational elasticity of the enzyme is affected by immobilization. It is often found that immobilized enzyme has a higher thermal stability than that of free enzyme because of restriction of conformational elasticity in immobilized enzyme [23, 24].
Repeated use capability
The operational stability of immobilized enzyme systems is one of the most important criteria for various biotechnological and biomedical applications; an increased stability could make the immobilized enzyme more advantageous than its free counterparts. Immobilized urease by using C-A PEC and P(AAm-co-AA)/carrageenan were used repeatedly 20 and 30 times within 5 days, respectively. Measured relative activities are presented in Fig. 6. It was observed that, after 20th use, immobilized urease into C-A PEC retained 55.0 of its original activity. And also, the retained activity for P(AAm-co-AA)/carrageenan immobilized urease was found to be 80.0% in the same use frame. Immobilized enzymes activities decreased while reuse number increased. These results could be explained by the inactivation of the enzyme caused by the denaturation and the leakage of enzyme from gels upon use and diffusional effects [23, 24].
Conclusion
In the present study, Jack bean urease was immobilized by using chitosan–alginate polyelectrolyte complexes and IPNs of poly(acrylamide-co-acrylic acid)/κ-carrageenan. These polymer matrixes have certain advantages over other materials such as easy preparation procedure, low cost, high immobilization capacity, and good mechanical stability for various biotechnological and biomedical applications.
The optimum pH for free and both immobilized urease were observed to be 7.5 and 8.0, respectively. The optimum temperature for free and immobilized enzymes into C-A PEC and P(AAm-co-AA)/carrageenan were also found to be 55, 60, and 55 °C, respectively. Thermal stability of the urease was increased upon immobilization. Upon 70 days of storage the preserved activity of the C-A PEC and P(AAm-co-AA)/carrageenan immobilized enzyme was 48 and 70% of its original activity. Also, high reusability was obtained for immobilized ureases.
References
Zerger B (1991) Bioorg Chem 19:116–131
Chang TMS (1980) Enzyme Eng 5:225–229
Krajewska B, Leszko B, Zaborska W (1989) Environ Prot Eng 15:173–180
George S, Chellapandian M, Sivasankar B, Jayaraman K (1997) Bioprocess Eng 16:83–85m
Elçin YM (1995) Biomaterials 16:157–1161
Chen PJ, Chiu SH (2000) Enzyme Microb Technol 26:359–367
Loke WK, Lau SK, Yong LL, Khor E, Sum CK (2000) J Biomed Mater Res 53:8–17
Hari PR, Candy T, Sharma CP (1996) J Appl Polym Sci 59:795–1801
Liu LS, Liu SQ, Ng SY, Froix M, Ohno T, Heler J (1997) J Controlled Release 43:65–74
Polk A, Amsden B, Yao DK, Peng T, Goosen MFA (1994) J Pharm Sci 83:178–185
Lipotov SY (2002) Prog Polym Sci 27:1721–1801
Bajpai AK, Bajpai P, Shukla S (2001) React Funct Polym 50:9–21
Yeon KH, Leueptow RM (2006) J Chem Technol Biotechnol 81:940–950
Karakus E, Pekyardimci S, Kilic E (2006) Process Biochem 41:1371–1377
Prakash O, Lipadhyay LSB (2006) Biotechnol Bioprocess Eng 11:140–145
Kuralay F, Ozyoruk H, Yildiz A (2006) Sensor Actuat B Chem 114:500–506
Reddy KRC, Kayasta AM (2006) J Mol Catal B Enzym 38:104–112
Hearn E, Nevfeld RJ (2000) Process Biochem 35:1253–1260
Kayastha AM, Srivastava PK (2001) Appl Biochem Biotechnol 96:41–53
Qin Y, Cabral JMS (1994) Appl Biochem Biotechnol 49:217–240
Kayastha AM, Das N (1999) Biochem Educ 27:114–117
Vaillant F, Millan A, Millan P, Dornier M, Decloux M, Reynes M (2000) Process Biochem 35:989–996
Yahşi A, Şahin F, Tümtürk H, Demirel G (2005) Int J Biol Macromol 36:253–258
Şahin F, Demirel G, Tümtürk H (2005) Int J Biol Macromol 37:148–153
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kara, F., Demirel, G. & Tümtürk, H. Immobilization of urease by using chitosan–alginate and poly(acrylamide-co-acrylic acid)/κ-carrageenan supports. Bioprocess Biosyst Eng 29, 207–211 (2006). https://doi.org/10.1007/s00449-006-0073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-006-0073-0