Abstract
Little information is available in the literature concerning the kinetics of the production of exopolysaccharides (EPS). The kinetics of growth, substrate consumption and lasiodiplodan (LAS) production by the filamentous fungus Lasiodiplodia theobromae MMPI were studied in a new bioreactor design (Low-Shear Aerated and Agitated Bioreactor; LSAAB) using media based on glucose and glycerol. The kinetic parameters of cultivation were compared with fermentations conducted in a stirred tank bioreactor (STR) and in shake flasks. Cultivation in LSAAB using glucose resulted in 3.17 ± 0.16 g.L−1 of LAS, a value lower than that obtained in shake-flasks (6.49 ± 0.03 g.L−1), but somewhat higher than obtained in STR (0.70 ± 0.12 g.L−1). When glycerol was used as substrate, the production of LAS was less effective than glucose. From the fungal biomass produced, as well as the related growth kinetic parameters (YX/S and QX), fungal growth was more efficient in LSAAB. Lasiodiplodan production by L. theobromae MMPI from glycerol was accelerated by an increase in broth pH during cultivation, and the behavior was opposite (decreased pH) when using glucose. LSAAB proved to be promising for the production of LAS by the fungus studied. Although glucose was shown to be the better substrate for the production of LAS, glycerol, as a fermentation substrate, has potential of a less-expensive medium for cultivating the fungus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polysaccharides are natural biomacromolecules composed of sugar residues that possess peculiar physicochemical properties and biological functions, which can have diverse applications in food, industrial (pharmaceutical, cosmetics, chemicals) and agricultural sectors [1, 2]. They constitute an emerging market with projections of a global market value of around US $7.2 billion by 2024 [3]. In addition to these traditional uses, recent research highlights new applications for polysaccharides, especially in the field of pharmaceutical therapy and tissue engineering [2, 4,5,6,7].
A group of polysaccharides that has attracted attention in recent years are the β-D-glucans, and especially those of non-cellulosic origin, such as the (1 → 3)- β-D-glucans that are classified as biological response modifiers [8], and have peculiar technological and biological properties. The growth expectations for the global market of β-glucans appears promising considering the increased use of these polysaccharides as food supplements. Consumer preference over synthetic ingredients has driven the market that is estimated to reach a value of US $560.65 million by 2026 [9].
Exopolysaccharides (EPS) are polysaccharides secreted by some fungi and bacteria that, depending upon the microbial system, form capsules around the cell, integrating with the cell wall, or appear outside the cell wall in the form of a slime that diffuses into the extracellular environment [10,11,12,13]. Lasiodiplodan (LAS), an uncommon, linear (1 → 6)-β-D-glucan, is an EPS produced by Lasiodiplodia theobromae MMPI [14], a filamentous fungus characteristic of tropical regions and a pathogen of various plant species [15].
In the natural environment, (1 → 6)-β-D-glucans are normally found in the cell wall matrix of fungi and in members of the phylum Chromista [16], and can be isolated by procedures that involve sequential extractions with hexane, hot and cold water and alkalis [17]. However, in the submerged cultivation of L. theobromae MMPI, LAS is secreted into the culture medium, and can simply be isolated by precipitation with alcohol, an effective and inexpensive extraction method [18].
Besides the microorganism, the fermentation parameters that most influence the production of EPS are the composition of the nutrient medium, temperature, pH, agitation and aeration during the fermentation process. Such parameters have a direct influence on the quantity and functional properties of the exopolysaccharides obtained, and consequently, on their applications. Different carbon sources can be used in the fungal production of EPS, with glucose and sucrose being the most commonly used substrates [19]. The use of alternative raw materials as fermentation feedstocks, such as by-products from industrial chemical and biochemical processes and agro-industrial wastes, not only lower the cost of production, but also minimize environmental problems caused by improperly disposing of industrial waste products. Glycerol is an example of a low-cost and widely-available by-product generated from the production of biodiesel from plant seed oils, and has shown potential as a fermentation substrate to obtain various products of economic and environmental interest [20].
In the cultivation of EPS-producing microorganisms, one of the main problems is the lack of homogeneity inside the bioreactor, since during the process there is a significant increase in the viscosity of the culture medium [21]. In culture media where the fermentation broth exhibits this behavior and where shear-sensitive filamentous fungi are used, the use of conventional bioreactors such as the Stirred Tank Reactor (STR) does not appear to be suitable. In the STR, microbial cell adhesion to the impeller blades and to the air distribution tube holes prevents homogeneous air and nutrient distribution in the culture medium. In addition, agitation promoted by the impeller blades causes significant shear of the fungal mycelium and hyphae.
In order to overcome the disadvantages of conventional bioreactors, the Low-Shear Agitated and Aerated Bioreactor (LSAAB) was developed [22, 23]; a system that allows simultaneous aeration and agitation of the liquid medium, thus avoiding the obstruction of air outlets, which are in continuous motion, due to the larger diameter compared to the holes commonly used in conventional bioreactors air diffusers. It is also noteworthy that the distribution of bubbles in a continuous circular motion promotes additional agitation of the medium, thus contributing to the inhibition of clump formation and a reduction in shear rate [22]. The LSAAB was first tested by Domingos et al. [23] for the cultivation of the basidiomycete, Ceriporiopsis subvermispora. The authors confirmed the bioreactor’s efficiency as a function of the higher amount of biomass that can be produced and the smaller formation of chlamydospores by this filamentous fungus.
In this context, the objective of the present work was to study the growth, substrate consumption and the kinetics of lasiodiplodan production by the filamentous fungus Lasiodiplodia theobromae MMPI using glucose and glycerol in LSAAB, and compare these with data obtained by STR, the most commonly used bioreactor. Shake-flask cultivation was included for comparative purposes.
Material and Methods
Microorganism and Inoculum Preparation
A fungal strain of Lasiodiplodia theobromae MMPI isolated from the fruit pinha (Annona squamosa) was maintained on PDA (potato dextrose agar) at 4 °C, and sub-cultured at 3-monthly intervals [24]. Pre-inoculum was prepared by growing the fungal isolate on PDA plates at 28 °C for 96 h. With the aid of an inoculating loop, a small amount of mycelium and hyphae was scraped from the surface of agar plates colonized with freshly growing fungal mycelium, and transferred to 250 mL Erlenmeyer flasks containing 90 mL of Vogel minimum salts medium [25] and 10 mL of a 50 g.L−1 glucose solution. Although a lower concentration of glucose (5 g. L−1) was used to minimize the amount of EPS produced in preparing the pre-inoculum, this concentration was still sufficient to stimulate fungal mycelial growth. The flasks were placed in an orbital motion shaker (New Brunswick Scientific, Innova 4000, Edison, USA) for 48 h at 180 rpm and 28 °C. The biomass obtained was homogenized aseptically with the aid of a mixer for 30 s, and then centrifuged (1900×g for 30 min). The sedimented mycelium (biomass) was then resuspended and diluted with isotonic saline to make a suspension with an absorbance between 0.4 and 0.5 at 400 nm, as described by Steluti et al. [26] with adaptations.
Production of Lasiodiplodan by Lasiodiplodia theobromae MMPI in Shake Flasks
In this group of tests, the influence of the initial substrate concentration combined with the initial concentration of the nitrogen source (yeast extract) were evaluated on the production of LAS by L. theobromae MMPI.
Tests were performed in 250-mL shake flasks containing 45 mL culture medium and 5 mL inoculum (mycelial suspension prepared as described above). The operating conditions employed for fermentation were the same as those for the preparation of inoculum. Culture medium was prepared and consisted of: K2HPO4 (2 g.L−1) and MgSO4.7H2O (2 g.L−1) as sources of mineral salts; yeast extract (Sigma‐Aldrich, St. Louis, MO, USA) as nitrogen source; and glucose or glycerol as carbon source. Both the nitrogen and carbon sources were evaluated at different levels, as shown in Table 1. The initial pH was adjusted to 5.0 with 5 mol.L−1 HCl solution.
Experiments were performed in duplicate, and at every 24-h timepoint, two flasks were removed from the shaker as a sample. Fermentation was discontinued after 72 h. Samples were analyzed for the biomass produced, substrate consumption, LAS production and pH of the fermentation broth.
Lasiodiplodan Production by Lasiodiplodia theobromae MMPI in a Stirred Tank Reactor
L. theobromae MMPI was grown in a STR using the same concentrations of carbon source (glucose or glycerol) and yeast extract as in the shake flasks experiments that produced the highest amount of LAS.
The tests were carried out in a STR (BIOFLO III, New Brunswick Sc. Co., Edison, USA), working volume of 1.25 L and equipped with temperature, agitation and aeration controls. Fermentations were operated in batch mode using 1.0 L of medium volume, temperature of 28 °C, initial pH of 5.0, agitation of 400 rpm, and aeration of 0.8 vvm. Inoculation was performed using 50 mL of mycelial suspension obtained as described in item 2.1.
Lasiodiplodan Production by Lasiodiplodia theobromae MMPI in a Low-Shear Agitated and Aerated Bioreactor
L. theobromae MMPI was grown in a LSAAB using the same carbon source (glucose or glycerol) and yeast extract concentrations as in the STR tests, to compare fungal growth and the kinetics of LAS production in the two bioreactor types.
The LSAAB corresponds to a 14-L Marriott bottle (see Supplementary Material, Fig. 1), the top of which is fitted with a cap with specific characteristics as described in a patent [22]. The fermentation process was operated in a batch system employing 10 L of medium volume, initial pH of 5.0, agitation of 300 rpm and aeration of 0.8 vvm. The bioreactor was inoculated with 500 mL of mycelial suspension obtained as described in item 2.1. Samples (50 mL) were periodically collected from the lower outlet. The temperature was controlled using a TIC-17RGTi (Full Gauge Control, Canoas, Brazil) digital thermostat model, equipped with a thermistor type temperature sensor. The thermostat charge terminal was connected to an externally fixed electrical resistor on the LSAAB bottle. The collar-shaped resistance (built of stainless steel) was run at 110 V and at a power of 400 W. Its diameter is 23 cm, close to the outer diameter of the bioreactor bottle, and the width is 9.8 cm, having been fixed around the bioreactor using two bolts with nuts in the horizontal position, connecting its two ends. The thermostat temperature sensor was placed in contact with the fermentation broth by passing it through one of the bioreactor cap holes.
Analytical Methods
Biomass
The amount of mycelium produced was quantified gravimetrically as dry mass. Samples from the glucose fermentation tests were first diluted 1:2 to decrease their viscosity. The samples were next centrifuged for 30 min at 1900×g, and the mycelium recovered washed with deionized water. After washing, the mycelial samples were placed in porcelain crucibles and dried in an oven (FANEM/Model 315 SE, São Paulo, Brazil) to constant mass at 70 °C, and is recorded as the amount of mycelium (Px).
Lasiodiplodan
LAS quantification was performed by precipitating the EPS with absolute ethanol. Alcohol at −10 °C was added to the fermented broth in a ratio of 1:4 (medium volume: alcohol volume), and the mixture left for 24 h at 4 °C. After precipitation, the LAS was recovered by vacuum filtration on qualitative filter paper (Whatman #1). The filter paper containing the LAS precipitate was oven-dried at 70 °C until constant mass and was recorded as the LAS concentration (Pp).
Carbon Source Concentration and Lasiodiplodan Purity
Both glucose and glycerol concentrations in shake flasks were determined by high-performance liquid chromatography (HPLC; Waters, Milford, MA, USA) using a RI Detector (model 2414) equipped with a Biorad Aminex HPX-87H column (300 × 7.8 mm), and operated under the following conditions: column temperature of 45 °C, eluent sulfuric acid 0.005 mol.L−1, eluent flow rate 0.6 mL.min−1, and injection sample 20 μL. All samples were properly diluted and filtered using a Sep Pak C18 filter (MILLIPORE, USA), and the eluent prior to use, was filtered under vacuum on a 0.45 μm HAWP membrane (MILLIPORE), and degassed in an ultrasonic bath (THORNTON, Vinhedo, SP, Brazil) operating at 40 kHz) for 15 min. Glycerol concentrations in the bioreactors (STR and LSAAB) was also quantified by HPLC.
Glucose in bioreactors (STR and LSAAB) was determined by the 3,5-dinitrosalicylic acid (DNS) method according to Miller [27] with subtle modifications.
The purity of LAS samples was estimated by determining the content of total carbohydrates by the phenol–sulfuric method [28], and proteins by the Bradford method [29].
Determination of Fermentation Kinetic Parameters
The kinetics of the performance of the fungus during the fermentation runs evaluated growth (measured as biomass), substrate consumption, pH variation and LAS production by L. theobromae MMPI in submerged liquid culture.
The following kinetic parameters were calculated according to Eqs. 1–7 below:
(a) Conversion yield of substrate to cell biomass (YX/S)
(b) Conversion yield of substrate to LAS (YP/S)
(c) Specific yield (YP/X)
(d) Percentage of consumed substrate (YC)
(e) Biomass volumetric productivity (QX)
(f) LAS volumetric productivity (QP)
(g) Global rate of substrate consumption (QS)
Statistical Analysis
Values of the kinetic parameters determined in fermentations conducted in the STR and in the LSAAB were compared with each other by the Student’s t test. Anova and Tukey’s test were used to compare data on the production of fungal biomass and LAS in the different nutritional conditions of cultivations conducted in shake flasks. Statistica 7.0 software was used in the analyzes, and were considered at the 95% confidence level (p < 0.05).
Results and Discussion
Shake Flask Fermentation Tests to Define Substrate and Nitrogen Concentrations
Fermentation experiments were firstly performed in shake flasks in liquid submerged cultivation to define adequate concentrations of glucose, glycerol and yeast extract for the production of LAS by L. theobromae MMPI.
Glucose as Substrate
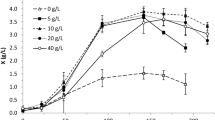
The results of the maximum amounts of biomass and LAS produced for fermentation tests A to I (experimental conditions—Table 1), in which glucose was used as carbon source, are shown in Fig. 1a. The condition of test G provided the highest amount of both biomass (31.74 ± 0.59 g.L−1) and LAS (6.49 ± 0.03 g.L−1), indicating a correlation between fungal growth and EPS production, as was also previously observed [18]. In addition, the fact that test G used the highest concentration of carbon source (50 g.L−1 glucose) and the lowest concentration of yeast extract (3 g.L−1) demonstrated that limiting concentrations of some nutrients in the presence of excess of carbohydrate appeared to favor exopolysaccharide production [13, 30]. This was confirmed by analyzing the tests in which the highest concentration of yeast extract (C, F and I) was used. Among the 50 g.L−1 glucose tests, test I resulted in the lowest concentration of LAS. A similar behavior was observed in tests with 30 g.L−1 glucose (D, E and F), where the lowest amount of LAS produced occurred under the condition of test F, where the highest concentration of nitrogen source was used (12 g.L−1).
Maximum values of the amounts of biomass and LAS obtained at 72 h by Lasiodiplodia theobromae MMPI in shake-flask cultivation at different concentrations of: a glucose and yeast extract; b glycerol and yeast extract (see Table 1 for experimental conditions)
The profile of fermentation from test G (highest biomass and LAS production) is shown in Fig. 2a, where the values of biomass, LAS, substrate concentrations and pH are included. After 48 h of cultivation there was little production of both biomass and LAS. Such stagnation could be attributed to the increased apparent viscosity of the medium, which, although not measured, was clearly observable. Consequently, there would be less mass transfer (mainly oxygen) in the culture medium, limiting both fungal growth and LAS production when compared with samples analyzed within the intervals: 0 to 24 h and 24 to 48 h. This statement is supported by the fact that at 48 h there was no substrate limitation in the medium. In addition, a low nitrogen supply (as in the case of test G) is considered favorable for EPS production, as reported by Nampoothiri et al. [31].
Kinetics of fungal growth, LAS production, substrate consumption and pH variation during shake-flask cultivation of Lasiodiplodia. theobromae MMPI in: (a) medium containing 50 g.L−1 glucose and 3 g.L−1 yeast extract (test G); and (b) medium containing 30 g.L−1 glycerol and 12 g.L−1 yeast extract (test O)
In the first 24 h of fermentation, 46% of the glucose present in the medium was consumed, while at the end of 72 h, only 2.5% of its initial concentration remained. Steluti et al. [26], in cultivating the fungus Botryosphaeria sp. MAMB-05 with 50 g.L−1 glucose, also observed a minimum amount of residual sugar in the medium at 72 h (2.4 ± 0.8 g.L−1). Cunha et al. [18], in the cultivation of L. theobromae MMPI using 40 g.L−1 glucose, observed that about 30% of the substrate remained in the fermentation broth at 120 h. In our case (test G), as was also reported by Cunha et al. [18] using the same carbon source, a possible explanation for the residual substrate difference was the higher concentration of nitrogen source used in test G of our study, which may have contributed to higher substrate consumption, but without resulting in a higher EPS production. It should be noted that the production of LAS observed by Cunha et al. [18] was 7.01 ± 0.07 g.L−1, a slightly higher value than the LAS produced from test G (6.49 ± 0.03 g.L−1).
Glycerol as Substrate
In Fig. 2b are shown the results from test O (using glycerol as carbon source). In this case, 30 g.L−1 glycerol and 12 g.L−1 yeast extract were employed, and produced the highest amount of LAS (3.39 ± 0.06 g.L−1). This behavior showed an important difference between the two substrates tested. With glycerol as carbon source, unlike that of glucose (30 g.L−1 and 50 g.L−1, respectively), the higher the concentration of nitrogen source used, resulted in the higher the amount of LAS produced (tests J, K, L; M, N, O and P, Q, R). This observation indicates that nitrogen limitation favored the production of LAS from glucose, but not in the case of glycerol. That is, when glycerol is used as the carbon source, both biopolymer production and fungal growth were favored by an increase in the nitrogen concentration, with one exception (test K, in which fungal growth decreased as the yeast extract concentration increased from 3 g.L−1 to 6 g.L−1). Considering the tests using equal carbon source concentrations, highest amounts of biomass were obtained at the highest nitrogen concentration used (tests L, O and R). Thus, it can be concluded that cultivation of L. theobromae MMPI on nutrient media containing glycerol as substrate requires a higher concentration of nitrogen, both for better production of fungal biomass and LAS, when compared to glucose-containing media (Fig. 1a). Torres et al. [32] studied the impact of glycerol and nitrogen concentration on EPS production by Enterobacter A47. The authors also concluded that an increase of initial nitrogen concentration (diammonium phosphate) promoted higher bacterial cell growth. However, unlike tests J to R, a higher concentration of nitrogen source impaired the synthesis of LAS. In view of the foregoing, it seems reasonable to assume that the synthesis of LAS is governed by factors not yet fully understood, but which differ from one microorganism to another, as well as according to the carbon sources employed in the bioprocess.
In the case of test O, both the highest mycelial growth and biopolymer production occurred within 48 h, with both values remaining practically constant up to 72 h. The highest glycerol consumption observed occurred within 24 to 48 h, during the same period coinciding with highest biomass production. Unlike the tests using glucose, total substrate consumption was not observed using glycerol as the carbon source. The condition of test J was the one that favored highest glycerol consumption (76.32%), and that of test R provided the lowest consumption (22.36%), although resulting in highest mycelial growth (15.47 ± 0.5 g.L−1). This observation suggests that the cultivation/production of LAS from glycerol by L. theobromae MMPI required a lower concentration of carbon source as compared to glucose.
The pH varied differently for each substrate used, indicating a different metabolic activity in relation to the consumption of the carbon source (in this case, glucose and glycerol) by the fungus. Except for tests A and B, glucose media showed a drop in pH up to 24 h of fermentation, followed by an increase up to 48 h (see Supplementary Material, Fig. 2a). The condition of test G, in which the highest accumulation of both fungal biomass and LAS was observed, provided the greatest pH drop (from 5.0 to 3.75) during the first 24 h. In addition, the final pH was lower (4.56) than the initial one (5.0). Steluti et al. [26] observed a similar behavior by cultivating Botryosphaeria sp. MAMB-05 on glucose media at different initial concentrations (25 g.L−1, 50 g.L−1 and 75 g.L−1), and the largest reduction in final pH was observed in the test with 75 g.L−1, which showed the highest growth of the fungus. Similarly, Lazaridou et al. [33] reported that in the production of pullulan by Aureobasidium pullulans on sugarbeet molasses, the pH of the medium fell within the first 24 h of fermentation, and then subsequently increased. The authors attributed such a pH rise to the deamination of amino acids and the production of ammonia due to depletion of the nitrogen source. Thus, in the case of the present study, it is possible to infer that the same phenomenon may have occurred with L. theobromae MMPI, considering that the medium of test G was the one that contained the lowest concentration of nitrogen source (Table 1).
Contrary to what was observed with glucose, in all tests using glycerol there was an increase in pH within the first 24 h of cultivation to 7.0 (Supplementary Material, Fig. 2b). The increase in pH may have resulted from the selective absorption of some nutrients from the culture medium by the fungus as suggested by Barroso and Nahas [34]. This fact suggests that such nutrients are necessary for metabolism of glycerol, but not for glucose. Between 24 and 72 h, except for tests L and K, there was a drop in pH. At the end of 72 h, the pH of all culture media showed higher values than the initial pH (5.0). Steluti et al. [26] tested 8 carbon sources (glucose, fructose, mannose, galactose, mannitol, sorbitol, lactose and sucrose) for the cultivation of Botryosphaeria sp. MAMB-05 in shake flasks and found that only culture media with sugarcane molasses and galactose presented a final pH value higher than the initial pH.
The opposing profiles of pH variation between cultivation on glucose and glycerol media, especially in the first 24 h of fermentation, corroborates what was previously suggested, regarding the fact that the process is not completely understood, differing clearly as a function of the nature of the carbon source employed.
Fermentation in the STR and LSAAB Reactors with Glucose as Substrate
The fermentation conditions of test G (50 g.L−1 glucose and 3 g.L−1 yeast extract) conducted in the shake flasks revealed the best results for LAS production, and was, therefore, the culture medium chosen for the fermentation runs in STR and LSAAB. Cultivation was performed as described in items 2.3 and 2.4.
The profiles of fungal growth, glucose consumption and LAS production during the cultivation of L. theobromae MMPI in STR and LSAAB are shown in Fig. 3a and b, respectively.
Fermentation in a STR
The highest amount of fungal biomass produced in STR was 5.08 ± 0.71 g.L−1 at 28 h of fermentation. In test G carried out in shake flasks, whose medium contained the same composition, the maximum fungal biomass produced was 31.74 ± 0.59 g.L−1 and occurred at 72 h. Therefore, the growth in STR was much lower than in shake flasks. Cunha et al. [18] obtained fungal biomass of 11.9 ± 0.57 g.L−1 using 40 g.L−1 glucose medium at 120 h in a STR. The authors also observed that the fungal biomass obtained in the STR was lower than that obtained in shake flasks (17.3 ± 0.8 g.L−1).
The highest concentration of LAS obtained in STR (0.70 ± 0.12 g.L−1) was much lower than that in shake flasks (6.49 ± 0.03 g.L−1). Cunha et al. [18] obtained fungal biomass of 11.9 ± 0.57 g.L−1 and 5.70 ± 0.16 g.L−1 of LAS using 40 g.L−1 glucose medium at 120 h in a STR. The authors also observed that the fungal biomass and LAS obtained in the STR were lower than that obtained in shake flasks (17.2 ± 1.13 g.L−1 and 7.01 ± 0.07 g.L−1 respectively). The authors attributed the lower fungal growth and LAS production of STR to low oxygen transfer due to the increased viscosity of the medium associated with intense mycelial growth. As observed by Cunha et al. [18], in the experiments carried out at STR, the fungus clung to the baffles, impeller blade, bioreactor walls and lid, forming large mycelial clumps (Fig. 4, panels e and f). At 18 h of cultivation, the fermentation medium became considerably viscous (visual observation) and there was virtually no circulation of the culture medium, with only some circulation observed at the bottom of the reactor near the impeller area. The lack of homogeneity compromised cultivation, as the biomass and LAS produced, as well as the pH value, remained practically constant from 18 h onwards. At 28 h, it was no longer possible to take a sample, as there was no apparent circulation of the medium due to the viscous nature, and the fermentation appeared to have terminated (Fig. 4, panel d). The fungus probably had not yet reached the end of the logarithmic growth phase or early stationary growth phase; stages of the largest production of exopolysaccharides by fungi [35]. This fact suggests that the fermentation medium and the initial conditions were adequate, but the changes resulting from the fungus itself made the process unfeasible. In other words, after that time the lack of homogeneity in the fermentation broth made it impossible for the fungus to access the nutrients, as well as the oxygen required for growth and LAS production.
Images of the culture medium of Lasiodiplodia theobromae MMPI during cultivation in STR on 50 g.L−1 glucose and 3 g.L−1 yeast extract. a—initial time (on inoculation); b at 4 h; c at 18 h; d at 28 h; panels (e) and (f) show the fungus adhering to the baffles, impeller blade, bioreactor walls and lid within the bioreactor forming large mycelial clumps
As noted in Fig. 3a in the STR during the first hours of fermentation, there was a decrease in pH, as was similarly observed in the tests on shake flask fermentations with glucose as substrate. At 18 h the lowest pH value observed was 3.92. Similarly, the lowest pH observed in the tests of the shake flask experiments was 3.75 at 24 h of cultivation (Fig. 2a). There was no increase in pH beyond 24 h, as observed in the shake flasks tests, possibly due to the same factors that led to stagnation of substrate consumption.
The calculated kinetic parameters in STR are presented in Table 2. The rate of substrate consumption (Qs) in the STR was higher (0.98 ± 0.01 g.L−1.h−1) than that obtained by Cunha et al. [18] (0.23 ± 0.002 g.L−1.h−1), where L. theobromae MMPI was also cultivated in a STR on glucose (40 g.L−1) as substrate. Possibly, under the fermentation conditions studied in the STR, the metabolism of other substances may have been prioritized over fungal growth, and especially, the production of LAS, since at 24 h, glucose had been consumed to the extent of 48.8%, which was similar to that observed in shake flask cultivation (45.9%), also at 24 h of cultivation, but in which the production of biomass and LAS were much higher. In this case, the shearing action by the impeller blades of the bioreactor is considered an important condition that does not exist in shake flask cultivation.
Fermentation in a LSAAB
The highest concentration of fungal biomass achieved in the LSAAB was 7.95 ± 0.35 g.L−1, and occurred at 64 h of fermentation. Likewise, the maximum amount of LAS produced (3.17 ± 0.16 g.L−1) occurred at 68 h of cultivation. Despite the amount of LAS produced in the LSAAB being more than four times higher than that obtained in the STR test (0.7 ± 0.12 g.L−1), the results still correspond to half the amount obtained in shake-flask cultivation (6.49 ± 0.03 g.L−1).
Shortly after 68 h, the amount of LAS produced dropped slightly, indicating the possibility that LAS was consumed as an energy source during fermentation by the fungus. Giese et al. [36] reported that when Botryosphaeria rhodina MAMB-05 was grown on nutrient medium containing botryosphaeran (a (1 → 3)(1 → 6)-β-glucan) as substrate and a low concentration of glucose added to stimulate fungal growth, the fungus was able to produce enzymes (β-1,3-glucanases) that were associated with the degradation of botryosphaeran during the late stationary phase of growth, providing an energy source for the fungus to continue growing until the death phase. Our study therefore reinforces the hypothesis that the fungus L. theobromae MMPI can also use LAS as a carbon source.
The amount of LAS produced and the pH profile in the LSAAB allows us to infer that the onset/acceleration of production of LAS by L. theobromae MMPI grown on glucose is associated with some metabolic activity that causes a pH decrease in the fermentation broth. Figure 3b shows that the production of LAS commenced around 6 h and reached its highest level around 36–40 h, while the pH decreased from 5.0 at 6 h to 3.69 around 36 h of cultivation. The pH decreased was also observed in both the STR and shake-flask experiments. The lowest pH value observed in the LSAAB was 3.17 occurring at 64 h of fermentation, and then the pH value increased slightly. Thus, the pH behavior in the three different fermentation systems (shake flask, STR and LSAAB) proves that the metabolic pathway of glucose utilization by L. theobromae MMPI leads to a decrease in pH, independent of the fermentation system of this bioprocess.
The kinetic parameters for the fermentation of glucose in LSAAB are presented in Table 2. YX/S (0.18 ± 0.02 g.g−1) and QX (0.12 ± 0.01 (g.L−1.h−1) values in the LSAAB were lower than those observed in the STR, while YP/S (0.07 ± 0.001 g/g) and QP (0.04 ± 0.002) were higher, indicating that the STR system was more favorable for biomass production, while the LSAAB favored the production of LAS. The YP/X value (0.40 ± 0.04 g.g−1) was almost 3 times higher than the STR value. A high YP/X interestingly demonstrates a higher capacity for the production of LAS per gram of fungal biomass. As the main difference between the two bioreactors is related to shear, the parameter values mentioned suggest that such phenomena are most important in the production phase of LAS by L. theobromae MMPI, i.e., a lower shear led to better LAS production (LSAAB), while higher shear led to lower LAS production (STR), regardless of the amount of fungal biomass formed. This proposition is further supported by the fact that in shake-flask cultivation, where no shear exists under the conditions used, highest LAS production resulted among the three fermentation cases studied (shake flask, STR and LSAAB).
In the LSAAB, the fungus adhered to the temperature sensor and the defoamer injection hose without compromising the growth and homogeneity of the medium. At the end of the fermentation period, the medium appeared more homogeneous, without the formation of agglomerates that impair mass transfer. Unlike that observed in the STR, the LSAAB was able to maintain homogeneity of the culture broth, which favored the fungal isolate to grow well consuming substrate and producing LAS until the end of the cultivation period.
Fermentation in STR and LSAAB Reactors with Glycerol as Substrate
Among the shake flask tests conducted with glycerol, test O with 30 g.L−1 glycerol and 12 g.L−1 yeast extract, presented the best results for the production of LAS. These conditions were therefore chosen for the cultivation of L. theobromae MMPI in STR and LSAAB. The cultivations were performed as described in items 2.3 and 2.4.
The profile for fungal growth, glycerol consumption and LAS production, as well as the pH profiles for STR and LSAAB are shown in Fig. 5.
Fermentation in STR
The highest amount of biomass (11.4 ± 0.87 g.L−1) was observed at 60 h of fermentation. This result was similar to that observed in the shake-flask experiments at 60 h (11.22 ± 0.34 g.L−1) under the same culture conditions (test O). After 60 h, there was a drop in the amount of biomass produced, which coincided with the time when glycerol was completely consumed, and the fungal isolate entered the late stationary phase of growth.
The maximum amount of LAS produced was 3.02 ± 0.19 g.L−1, and occurred at 48 h of cultivation. After 48 h, the amount of LAS produced fell slightly, as was also observed in the LSAAB experiment with glucose as the substrate, indicating that the fungus possibly consumed some of the EPS produced for energy purposes. As observed for fungal growth, the highest amount of LAS produced was very similar to that obtained in shake flasks (3.39 ± 0.06 g.L−1) at 48 h of cultivation.
The kinetic parameters arising from the STR experiment in which glycerol was used as substrate are shown in Table 3. Although the final amounts of biomass and LAS produced by cultivation in STR were relatively close to those obtained in the shake-flask experiments, in the STR the fungus was less efficient in producing biomass and LAS. In STR, glycerol was completely consumed within 60 h of cultivation (Fig. 5a), while in shake flasks the substrate consumed was approximately half of the initial value (54.16%) supplied to the fungal cultures.
As with the shake flasks experiments, the pH in STR increased during the first hours of fermentation with glycerol as substrate, as opposed to the glucose-run fermentations in shake flasks, STR and LSAAB. However, the maximum pH values found in the STR (6.25) were lower than that observed in the shake flask tests (7.27). The phenomenon of pH elevation followed by a decrease when L. theobromae MMPI was cultivated in medium containing glycerol as substrate has not been reported in the literature. This is a topic that needs further study, which may even involve the dosing of metabolites other than EPS. This phenomenon seems to be related to the onset/acceleration of LAS production and deceleration of growth, as observed during the period 12 to 36 h in the STR. Such a hypothesis is supported by a similar behavior observed in the shake-flask tests, specifically with test run O (Fig. 2b).
In the STR, as well as in shake flasks, the fungal isolate showed filamentous and dispersed growth, and clung to the baffles, impeller, and bioreactor walls.
Fermentation in LSAAB
The highest amount of fungal biomass observed in the LSAAB was 28.86 ± 1.46 g.L−1 at 30 h of fermentation. This value was much higher than the biomass obtained in shake flasks (11.22 ± 0.34 g.L−1) and STR (11.40 ± 0.87 g.L−1) cultivations. The higher homogeneity, and consequently, greater aeration of the medium in the LSAAB favored biomass production, but not LAS, as the maximum amount of LAS produced was low, 1.45 ± 0.25 g.L−1.
The kinetic parameters of the fermentation experiment in the LSAAB are presented in Table 3. Comparing the values with those obtained in STR, the YX/S and QX values were higher, confirming that LSAAB was more suitable for fungal biomass production. On the otherhand, the YP/S, YP/X and QP values were lower, which means, unlike when glucose was used as substrate, that LAS production was not higher, or more efficient, in the LSAAB.
The production of LAS commenced at 16 h fermentation and continued to increase up to 40 h, while the pH increased from 16 to 30 h of cultivation (Fig. 5b), reinforcing what was suggested regarding the pH variation associated with the onset/acceleration of EPS production. As with the shake-flask experiments, the pH was observed to increase during the first 30 h of fermentation, with the highest pH value observed at 6.03, a value slightly lower than that observed in STR (6.25).
In the LSAAB, apart from the fungus adhering only to the temperature sensor during the fermentation run, the broth had a homogeneous appearance without formation of clumps.
Comparing the tests performed in LSAAB on the two substrates (glucose and glycerol), higher production of LAS resulted on the glucose-containing nutrient medium, and this was associated with an increase in the availability of oxygen in the culture broth. There was an increase in the production of LAS in LSAAB compared to the STR fermentation experiments. In this case, it is noteworthy that higher oxygen transfer in LSAAB was due to the greater homogeneity of the medium throughout the fermentation process.
Conclusion
Submerged cultivation of Lasiodiplodia theobromae MMPI in nutrient medium containing glucose or glycerol as substrate allowed the production of (1 → 6)-β-D-glucan (LAS) in shake flasks, stirred tank reactor and in a low-shear aerated and agitated bioreactor.
Glucose proved to be the best carbon source for both cell growth and LAS production in all of the fermentation conditions. Glycerol also demonstrated potential as a substrate for cultivating the fungus for the production of LAS, which is worthy of attention, since glycerol can be obtained at low cost as a by-product of industrial production of biodiesel from plant seed oils.
LSAAB showed better performance for mycelial growth and LAS production compared to STR with glucose as substrate, which suggests that the effects of shear during cultivation may be one of the bottlenecks for greater fungus performance in cultures normally carried out in bioreactors.
The results of the kinetic study of cultivation suggest that culture media with glycerol as carbon source requires a higher concentration of nitrogen (yeast extract), to provide both higher mycelial biomass and LAS production by L. theobromae MMPI compared to media containing glucose. The onset/acceleration of LAS production by L. theobromae MMPI, both on glucose and glycerol, appears to be related to the metabolic activity of the fungal isolate, which resulted in a variation of the pH of the culture medium that decreased when the substrate was glucose, but increased when the substrate was glycerol.
In the LSAAB cultivation experiments, the culture media remained homogeneous and without the formation of clumps, confirming that LSAAB is suitable for cultivating shear-sensitive microbial cells, and cells that have the inconvenience of easily adhering to the internal prominent parts of conventional bioreactors. In glucose medium, the LSAAB provided a significant increase in LAS production by the fungus under study compared to the STR experiments.
All lasiodiplodan samples showed a high degree of purity (97% carbohydrate, 2% of associated protein, and a mineral residue content of ~ 1%) regardless of the substrate used, or the configuration of the bioreactor wherein the fermentations were conducted.
References
Thombare N, Jha U, Mishra S, Siddiqui MZ (2016) Guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol 88:361–372. https://doi.org/10.1016/j.ijbiomac.2016.04.001
Kumar A, Rao KM, Han SS (2018) Application of xanthan gum as polysaccharide in tissue engineering: a review. Carbohydr Polym 180:128–144. https://doi.org/10.1016/j.carbpol.2017.10.009
Nagi M (2018) Global Biopolymers market to witness a CAGR of 15.2% during 2018–2024: Energias market research Pvt. Ltd. https://www.globenewswire.com/news-release/2018/07/11/1535796/0/en/Global-Biopolymers-Market-to-witness-a-CAGR-of-15-2-during-2018-2024-Energias-Market-Research-Pvt-Ltd.html. Accessed 17 Dec 2019
Baldassano S, Accardi G, Vasto S (2017) Beta-glucans and cancer: the influence of inflammation and gut peptide. Eur J Med Chem 142:486–492. https://doi.org/10.1016/j.ejmech.2017.09.013
Bozbulut R, Sanlier N (2019) Promising effects of β-glucans on glycemic control in diabetes. Trends Food Sci Technol 83:159–166. https://doi.org/10.1016/j.tifs.2018.11.018
Han F, Fan H, Yao M et al (2017) Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J Funct Foods 35:115–126. https://doi.org/10.1016/j.jff.2017.05.036
Orlandelli RC, Corradi da Silva M, de L, Vasconcelos AFD, et al (2017) β-(1→3,1→6)-D-glucans produced by Diaporthe sp. endophytes: Purification, chemical characterization and antiproliferative activity against MCF-7 and HepG2-C3A cells. Int J Biol Macromol 94:431–437. https://doi.org/10.1016/j.ijbiomac.2016.10.048
Bohn JA, BeMiller JN (1995) (1→3)-β-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polym 28:3–14. https://doi.org/10.1016/0144-8617(95)00076-3
Grand View Research (2020) Beta-glucan Market Size, Share & Trends Analysis Report By Source (Cereal, Mushroom, Yeasts, Seaweed), By Application (F&B, Personal Care, Pharmaceuticals, Animal Feed), By Type, By Region, And Segment Forecasts, 2018 - 2025. https://www.grandviewresearch.com/industry-analysis/beta-glucan-market. Accessed 17 Jun 2020
Osińska-Jaroszuk M, Jarosz-Wilkołazka A, Jaroszuk-Ściseł J et al (2015) Extracellular polysaccharides from Ascomycota and Basidiomycota: production conditions, biochemical characteristics, and biological properties. World J Microbiol Biotechnol 31:1823–1844. https://doi.org/10.1007/s11274-015-1937-8
Kagimura FY, Da Cunha MAA, Theis TV et al (2015) Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): preparation, characterization and antioxidant evaluation. Carbohydr Polym 127:390–399. https://doi.org/10.1016/j.carbpol.2015.03.045
Decho AW (1990) Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Ann Rev 28:73–153
Sutherland IW (1979) Microbial exopolysaccharides. Trends Biochem Sci 4:55–59. https://doi.org/10.1016/0968-0004(79)90262-7
Vasconcelos AFD, Monteiro NK, Dekker RFH et al (2008) Three exopolysaccharides of the β-(1→6)-D-glucan type and a β-(1→3;1→6)-D-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit. Carbohydr Res 343:2481–2485. https://doi.org/10.1016/j.carres.2008.06.013
Úrbez-Torres JR, Leavitt GM, Guerrero JC et al (2008) Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of Grapevines in Mexico. Plant Dis 92:519–529. https://doi.org/10.1094/PDIS-92-4-0519
Fesel PH, Zuccaro A (2016) β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol 90:53–60. https://doi.org/10.1016/j.fgb.2015.12.004
Corradi da Silva M, de L, Fukuda EK, Vasconcelos AFD, et al (2008) Structural characterization of the cell wall D-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05. Carbohydr Res 343:793–798. https://doi.org/10.1016/j.carres.2007.12.021
Cunha MAA, Turmina JA, Ivanov RC et al (2012) Lasiodiplodan, an exocellular (1→6)-β-D-glucan from Lasiodiplodia theobromae MMPI: Production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J Ind Microbiol Biotechnol 39:1179–1188. https://doi.org/10.1007/s10295-012-1112-2
Seviour RJ, Stasinopoulos SJ, Auer DPF, Gibbs PA (1992) Production of pullulan and other exopolysaccharides by filamentous fungi. Crit Rev Biotechnol 12:279–298. https://doi.org/10.3109/07388559209069196
Abad S, Turon X (2012) Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: Focus on polyunsaturated fatty acids. Biotechnol Adv 30:733–741. https://doi.org/10.1016/j.biotechadv.2012.01.002
Seviour RJ, McNeil B, Fazenda ML, Harvey LM (2011) Operating bioreactors for microbial exopolysaccharide production. Crit Rev Biotechnol 31:170–185. https://doi.org/10.3109/07388551.2010.505909
Prata AMR, Ferraz AL, Domingos M, Silva Junior JM, 2011. Biorreator com sistema de agitação e aeração específico para cultivo de células aderentes e/ou sensíveis ao cisalhamento. BR Patent PI 1003119–7I.
Domingos M, de Souza-Cruz PB, Ferraz A, Prata AMR (2017) A new bioreactor design for culturing basidiomycetes: mycelial biomass production in submerged cultures of Ceriporiopsis subvermispora. Chem Eng Sci 170:670–676. https://doi.org/10.1016/j.ces.2017.04.004
Saldanha RL, Garcia JE, Dekker RFH et al (2007) Genetic diversity among Botryosphaeria isolates and their correlation with cell wall-lytic enzyme production. Brazilian J Microbiol 38:259–264. https://doi.org/10.1590/S1517-83822007000200013
Vogel HJ (1956) A convenient growth medium for Neurospora crassa. Microbial Genet Bull 13:42–43
Steluti RM, Giese EC, Piggato MM et al (2004) Comparison of Botryosphaeran production by the ascomyceteous fungus Botryosphaeria sp., grown on different carbohydrate carbon sources, and their partial structural features. J Basic Microbiol 44:480–486. https://doi.org/10.1002/jobm.200410415
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem. https://doi.org/10.1021/ac60111a017
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Jarman TR, Pace GW (1984) Energy requirements for microbial exopolysaccharide synthesis. Arch Microbiol 137:231–235. https://doi.org/10.1007/BF00414549
Nampoothiri KM, Singhania RR, Sabarinath C, Pandey A (2003) Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem 38:1513–1519. https://doi.org/10.1016/S0032-9592(02)00321-7
Torres CAV, Marques R, Ferreira ARV et al (2014) Impact of glycerol and nitrogen concentration on Enterobacter A47 growth and exopolysaccharide production. Int J Biol Macromol 71:81–86. https://doi.org/10.1016/j.ijbiomac.2014.04.012
Lazaridou A, Roukas T, Biliaderis CG, Vaikousi H (2002) Characterization of pullulan produced from beet molasses by Aureobasidium pullulans in a stirred tank reactor under varying agitation. Enzyme Microb Technol 31:122–132. https://doi.org/10.1016/S0141-0229(02)00082-0
Barroso CB, Nahas E (2005) The status of soil phosphate fractions and the ability of fungi to dissolve hardly soluble phosphates. Appl Soil Ecol 29:73–83. https://doi.org/10.1016/j.apsoil.2004.09.005
Mahapatra S, Banerjee D (2013) Fungal exopolysaccharide: production, composition and applications. Microbiol Insights 6:1–16. https://doi.org/10.4137/mbi.s10957
Giese EC, Dekker RFH, Scarminio IS et al (2011) Comparison of β-1,3-glucanase production by Botryosphaeria rhodina MAMB-05 and Trichoderma harzianum Rifai and its optimization using a statistical mixture-design. Biochem Eng J 53:239–243. https://doi.org/10.1016/j.bej.2010.10.013
Acknowledgements
The authors thanks to Universidade de São Paulo (USP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tabuchi, S.C.T., Martiniano, S.E., Cunha, M.A.A. et al. Kinetic Study of Lasiodiplodan Production by Lasiodiplodia theobromae MMPI in a Low-Shear Aerated and Agitated Bioreactor. J Polym Environ 29, 89–102 (2021). https://doi.org/10.1007/s10924-020-01857-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01857-x