Abstract
Resource use by consumers across patches is often proportional to the quantity or quality of the resource within these patches. In folivores, such proportional use of resources is likely to be more efficient when plants are spatially proximate, such as trees forming a forest canopy. However, resources provided by forest-trees are often not used proportionally. We hypothesised that proportional use of resources is reduced when host trees are isolated among phylogenetically distant neighbours that mask olfactory and visual search cues, and reduce folivore movement between trees. Such phylogenetically distant neighbourhoods might sort out species that are specialists, poor dispersers, or have poor access to information about leaf quality. We studied individual oaks, their leaf size and quality, their folivory and abundance of folivores (mostly Lepidopteran ectophages, gallers and miners), and parasitism of folivores. We found that leaf consumption by ectophages hardly increased with increasing leaf size when host trees were phylogenetically isolated. We found a similar effect on host use by parasitoids in 1 year. In contrast, we found no consistent effects in other folivore guilds. Relative abundances of specialists and species with wingless females declined with phylogenetic isolation. However, resource use within each of these groups was inconsistently affected by phylogenetic isolation. We suggest that phylogenetic isolation prevents ectophages from effectively choosing trees with abundant resources, and also sorts out species likely to recruit in situ on their host tree. Trees in phylogenetically distant neighbourhoods may be selected for larger leaves and greater reliance on induced defences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
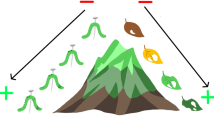
Resource use by a community of consumers and their natural enemies is often proportional to the quantity or quality of the resource (Holling 1959; MacArthur and Pianka 1966; Emlen 1966; Fretwell and Lucas 1969; Gripenberg et al. 2010). For example, folivory is higher on trees with larger leaves or higher leaf-nitrogen content (see Fig. 1 for conceptual representation, Moore and Francis 1991; Ruhnke et al. 2009). Such proportional use of resources by folivorous insects could result from ovipositing females or foraging caterpillars choosing resource-rich trees (Faeth et al. 1981; Cornelissen and Stiling 2008). In addition, individual folivores may be more reluctant to leave resource-rich trees than resource-poor trees (Charnov 1976), thereby increasing their abundance on resource-rich trees. Moreover, individually, folivores may consume more leaf area on trees with higher leaf quality. This is likely the case when individuals belong to species or genotypes that consume more (Wimp et al. 2004; Pilosof et al. 2017; Eisenring et al. 2021). Proportional use of resources is more likely when the trees are spatially close to each other, facilitating folivore movements between trees. But even resources provided by forest-trees are often not used proportionally (Faeth et al. 1981; Courtney and Kibota 1990; Gripenberg et al. 2007; Kitamura et al. 2007; Craig and Itami 2008), and factors that reduce proportional use of resources on forest trees remain poorly understood.
Proposed processes (top row) and the resulting pattern of proportional use of resources (bottom row) on phylogenetically non-isolated (left) and phylogenetically isolated trees (right). If phylogenetic isolation of an individual tree reduces the rate of movement of ovipositing adults or larvae of folivores that prefer large leaves, folivore pressure will correlate less with leaf size on more phylogenetically isolated tree
Forest trees can be phylogenetically separated from their spatial neighbours by millions of years of evolution (‘phylogenetic isolation’ from here on, Vialatte et al. 2010), and this may reduce proportional use of resources in folivores. Many folivores are specialised on one or a few usually closely related host plant species (Ehrlich and Raven 1964; Kennedy and Southwood 1984; Brändle and Brandl 2006; Seifert et al. 2020). Therefore, a larger number of folivores can be expected to move between neighbouring trees that are phylogenetically closely related than between those that are phylogenetically distantly related (Vialatte et al. 2010). Hence, when phylogenetic isolation of a tree is high, folivore movements between trees are expected to be scarce, compared to when phylogenetic isolation is low (Vialatte et al. 2010). In addition, phylogenetically distant neighbours could mask the olfactory and visual search cues of a focal tree (Castagneyrol et al. 2013; Binyameen et al. 2013), which may make the focal tree more difficult to detect for folivores (Jactel et al. 2011; Salazar et al. 2016). This can reduce the immigration of ovipositing females and foraging larvae (Jactel et al. 2011). In addition, this masking of search cues may cause folivores on phylogenetically isolated trees to be more reluctant to leave resource-poor trees (Charnov 1976; Stratton et al. 2019). Thus, both reduced movement and masking of search cues can reduce proportional use of resources (Fretwell and Lucas 1969). Consistently, more phylogenetically isolated trees have lower species richness and abundance of folivorous Heteropteran (Vialatte et al. 2010), and reduced leaf consumption by ectophagous lepidopteran caterpillars (Yguel et al. 2011). However, reduced abundance does not indicate reduction in proportional use of resources (Fretwell and Lucas 1969; Kratina et al. 2009; Beest et al. 2016; Avgar et al. 2020). To our knowledge, effects of phylogenetic isolation on proportional use of resources have never been studied.
Phylogenetic isolation may reduce proportional use of resources by sorting out folivore species with traits that facilitate it. Such traits could be (1) host plant specialisation, because specialised folivores use host resources more efficiently (Gripenberg et al. 2010; Charlery de la Masselière et al. 2017), (2) ovipositing when information on leaf quantity or quality is available, i.e., developed leaves are present, as this would facilitate the choice of large and good quality leaves, and (3) strong dispersal capacity, because better dispersers are better able to select the most profitable trees. Phylogenetic isolation may sort out some of these folivore traits. For example, when specialist folivores are more affected by phylogenetic isolation than generalists, and when weaker dispersers have a lower chance of finding phylogenetically isolated trees (as indicated by Vialatte et al. 2010 for Heteroptera). Specialist folivores could be more affected because they have lower chance to find phylogenetically isolated host tree as they are repelled by the odour from phylogenetically distant trees than their host tree species (Stratton et al. 2019). Moreover, optimal foraging theory (Charnov 1976) suggests that oak specialists will move less within forest stands where oaks are among phylogenetically distant trees than where oaks are among conspecifics. Thus, phylogenetic isolation may shape the species composition in folivore communities by sorting out species that would cause proportional use of resources.
Besides individual traits, folivores can be characterised by guild membership, such as ectophages, leaf miners and leaf gallers. Such guilds might differ in the effect of phylogenetic isolation on their degree of proportional use of resources, as they differ in capacity of active dispersal (Peterson and Denno 1998; Asplen 2018), and in perception and processing of information on leaf size and quality (Bernays and Funk 1999; Javoiš et al. 2019). More specialised guilds such as leaf miners and leaf gallers (Novotny et al. 2010) are on average better at choosing plants on which their larvae perform best (Gripenberg et al. 2010), which should lead to more proportional use of resources. Different guilds can also differ in their responses to leaf quality. For example, leaf miners can locally modify the leaf’s photosynthetic activity (Giron et al. 2007) and leaf gallers can modify the nutritional environment of their gall (Hartley and Lawton 1992), so that natural leaf quality is less relevant to them. Therefore, the ectophages that are poor at leaf modification might experience more selection to find host trees with large and high-quality leaves.
Parasitoids of folivores often have close relationships with particular plant species (De Moraes et al. 1998; Bailey et al. 2009) as they are usually specialised on one or a few folivore species (Schär and Vorburger 2013) which in turn are specialised on their host plants (Forister et al. 2015). Moreover, parasitoids can use the same olfactory and visual search cues as their host for finding the correct plants (Birkett et al. 2003; Aartsma et al. 2017). Therefore, the degree of phylogenetic isolation of the host tree might also affect proportional use of resources in parasitoids. This would be important because the parasitoids can partly regulate local folivore abundances, and thus affect the exploitation of leaf resources of a tree (Hunter and Price 1992). Parasitoids are often able to track hosts by exploiting plant volatiles over large distances (Gossner et al. 2014; Aartsma et al. 2017), and may thus be less affected by isolation than folivores. However, in the case of phylogenetic isolation, these volatile signals may be masked by volatiles of other plant species (Perfecto and Vet 2003), and this can cause reduced proportional use of resources in parasitoids. Moreover, given that a patch of resources is much smaller for the parasitoids (i.e. the folivores) than that of the folivores (i.e., the tree), parasitoids might also be more affected by phylogenetic isolation than folivores. Yguel et al. (2014) showed that overall parasitism rate of caterpillars tends to be lower on more phylogenetically isolated trees, but such low parasitism rate does not exclude proportional use of resources. Whether proportional use of resources in parasitoids is affected by phylogenetic isolation among trees has so far not been addressed.
We studied the effects of phylogenetic isolation of host trees on proportional use of resources in associated folivore guilds and parasitoids. As a model system, we used oaks (Quercus petraea (Matt.) Liebl, Q. robur L. and their hybrids) in a mixed forest and focused mainly on ectophages. We used a combination of raw data from earlier publications and unpublished data (Table 1). We tested the prediction that phylogenetic isolation reduces the strength of the relationship between resource quantity/quality and resource use, i.e. the proportional use of resources. We investigated three levels of resource use: per-leaf folivory, per-individual folivory and per-leaf abundance. At the level of per-leaf abundance, we also explored whether the effect of phylogenetic isolation of host trees on proportional use of resources is found in particular among species that possess traits that facilitate it: host-plant specialisation, ovipositing when leaves are present (inferred from flying from June to October), or high dispersal capacity (inferred from large wingspan or absence of wingless females). We then tested whether phylogenetic isolation per se affects overall species composition, and in particular if phylogenetic isolation sorts out species with traits that facilitate proportional use of resources. We finally expanded the tests of whether phylogenetic isolation reduces proportional use of resources to two more folivore guilds (leaf gallers and leaf miners, in terms of abundance) and to the parasitoids of ectophages (in terms of caterpillar use). For folivores, we characterised resource quantity as leaf area, and resource quality as leaf dry matter content, carbon–nitrogen ratio (C:N) and total leaf phenolic content. For parasitoids, we characterised resource quantity as the density of ectophagous caterpillars. We also tested how resource use becomes less proportional with increases in two more commonly studied variables, isolation of the resource patch in space (distance to nearest oak), and the age of the resource patch (age of oaks), and explored if phylogenetic isolation can be explained by the proportion of pines.
Methods
Field site and study trees
Our study was conducted in the Forest of Rennes (48° 11ʹ North, 1° 34ʹ West; c. 90 m altitude), a temperate forest in Western France of about 3000 hectares with an oceanic climate (mean annual temperature 11.3 °C; cumulative annual rainfall 836 mm). The forest is divided into 202 parcels that are usually either dominated by oaks (Q. petraea, Q. robur and their hybrids) or pines (Pinus sylvestris L.). We studied pairs of oaks (avoiding cross-species pairs) with trees within a pair being only 30–150 m apart, one in an oak-dominated parcel, and the other in a pine-dominated parcel. Neighbouring trees were from 19 species, spanning a continuous range of phylogenetic distances from oak (Online Resource 1, Table. S1). Pairs of study trees were spread across the entire forest. We studied the “First Set” of 9 pairs of adult oaks (producing acorns) in 2006 (earlier used in Vialatte et al. 2010; Yguel et al. 2011), and the “Second Set” of 11 pairs in 2010 and 2011 (earlier used in Yguel et al. 2011, 2014; see Table 1).
Neighbourhood and traits of study trees
For each study tree, we determined the degree of phylogenetic isolation, distance to the nearest oak, and circumference at breast height. In addition, crown position relative to dominant canopy and budburst phenology of each tree were recorded, as those are also known to affect folivore abundance and folivory (Crawley and Akhteruzzaman 1988; Eliason and Potter 2000; Castagneyrol et al. 2017; Barker et al. 2018; Ekholm et al. 2020; Faticov et al. 2020). Procedures and data sources are detailed below.
Degree of phylogenetic isolation for the First Set of trees had been calculated by Vialatte et al. (2010), and for the Second Set of trees by Yguel et al. (2011). Degree of phylogenetic isolation of each tree was calculated as mean phylogenetic distance to the trees with which its crown was in contact (Online Resource 1). Phylogenetic distances to all species of trees growing in contact with study trees were quantified as in Online Resource 1, Table S1. Understory trees (height < 6 m) were not considered because there is little exchange of insects between canopy and understory (Corff and Marquis 1999; Gossner et al. 2009).
Spatial distance from each study tree to the nearest adult oak was measured from trunk to trunk. This is not to be confused with the distance between two study trees in a pair. Rather, this distance is between one study tree and the nearest oak in its neighbourhood. Circumference of the study trees was measured at breast height. As larger trees tend to be older, the circumference is a good estimate of relative age of the trees for a given soil and macroclimate (Rozas 2003). According to local foresters, absolute age was on average ca. 80 years in the First Set, and on average ca. 60 years in the Second Set of trees.
Whether the crown position of study trees was within or below the dominant canopy was noted for the First Set of trees. Trees of the Second Set were always located below the dominant canopy. Budburst phenology was monitored only for the Second Set of trees, in 2010 and 2011. From the beginning of March, the developmental stage of 10 random apical leaf buds from the upper stratum was determined on a three-rank scale once every 3 days using binoculars (Wesołowski and Rowiński 2006). Every sampling day, 10 new buds were randomly selected. From these data we inferred the date when a tree completed budburst, i.e., all 10 random buds of the tree had developed unfolded leaves. We then set the date when the first tree completed budburst as 0, and calculated the budburst of other trees relative to this tree in days.
Quantity and quality of leaf resources
We measured leaf area as a parameter of resource quantity, and leaf dry matter content (LDMC), leaf carbon to nitrogen ratio (C:N) and total leaf phenolic content as parameters of resource quality (Feeny 1970; Forkner et al. 2004; Schoonhoven et al. 2005). For the First Set of trees, we collected 30 leaves per tree between 16th August and 27th September 2006: 10 leaves were collected from each of 3 strata (upper exposed to sun, lower exposed to sun, and lower sheltered), and the leaf trait measurements were averaged per tree. For the Second Set of trees in 2010, a total of 40 leaves (20 from upper and 20 from lower stratum) were sampled from each tree both in the beginning of May and in the middle of September. We used spring samples for leaf quality measurements, and autumn sample for leaf size measurements. This was done because leaf quality in September might differ drastically from what folivores experience in the beginning of Spring (Feeny 1970). Similar to 2010, 40 leaves were sampled from each tree in the middle of September in 2011, and used for leaf size measurement. In 2011, we did not sample leaves in spring and hence did not make any measurement of leaf quality.
Leaf area was estimated with a 1 × 1 cm2 dot grid and quantified as the number of dots covering the whole leaf (as described in Yguel et al. 2011). To estimate the size of the whole leaf we manually reconstructed the missing part that was eaten by the ectophages.
To measure leaf quality, the leaves were cut longitudinally into two pieces. The piece without the main central vein was used to analyse LDMC and C:N, while the larger piece was used to analyse leaf phenolics. LDMC analyses were made following the protocol of Cornelissen et al. (2003). C:N analyses were made by “flash combustion” using a Carlo Erba NA1500 Series II elemental analyzer for the First Set of trees, and a PerkinElmer CHN PE 2400 for the Second Set of trees. For the analyses of leaf phenolics, the leaves were frozen and lyophilised for 36 h, and pooled per stratum to obtain sufficient material. Leaf phenolics were characterised at the Polyphenols Biotech lab, Bordeaux (France), by spectrometry following the Folin–Ciocalteu indices method and expressed as the percentage of dry mass gallic acid equivalent (Singleton et al. 1999).
Folivore abundance and folivory
We studied the abundance of ectophages, leaf gallers and leaf miners to address the prediction developed in the Introduction. Abundance of ectophagous caterpillars on oaks peaks during the spring period (Southwood et al. 2004), and therefore we sampled caterpillars twice during the springs of 2010 and of 2011. The first sampling was done when all the study trees had just completed budburst, and the second was three weeks later. Each sample consisted of a two meters long branch, and from each study tree, one such sample was cut from the upper, and one from the lower stratum (both are sheltered). Samples were brought to the lab in plastic bags where the caterpillars were collected, and the leaves counted. To obtain per-leaf abundance of caterpillars, we divided the number of caterpillars collected from a tree by the number of leaves sampled. The leaf miners and leaf gallers are most abundant during the summer (West 1985), and hence we counted them from the leaves sampled for leaf area and folivory measurements in September.
Apart from counting individuals, we studied folivory caused by ectophages (Table 1). For this, we summed the values of missing leaf surface (see “Quantification of leaf resources”, see also Yguel et al. 2011) across all the leaves of a tree and divided that by the number of leaves to obtain per-leaf folivory. We then divided these summed values by the number of caterpillars to obtain per-individual folivory. We note that all our measures of folivory are absolute surfaces, not proportions of leaf area, given that leaf area was the independent variable in later analyses. Our aim was to understand under which conditions the absolute amount of leaf material used increased with quantity or quality of leaves available, rather than the percentage (as in Yguel et al. 2011).
Parasitism of ectophagous caterpillars
The ectophagous caterpillars collected were visually searched for ectoparasitoids. We then reared the caterpillars and monitored emergence of endoparasitoids for five months from the day of sampling. The caterpillars were reared individually in Petri dishes at ambient temperature and were fed every two days with fresh oak leaves. We used absolute numbers of parasitised caterpillars observed per tree, not proportions of a given number of caterpillars parasitized, given that number of caterpillars was the independent variable in later analyses. Again, our aim was to understand under which conditions the caterpillar use in terms of absolute number increases with the total number of caterpillars present, rather than the parasitism rate (as in Yguel et al. 2014).
Ectophages species identification
After collection, all caterpillars were photographed and assigned to morphospecies based on visual assessment. Those that were successfully reared to adults in the lab were identified to species. Those individuals that died before eclosion (due to parasitism or other causes) were assumed to be of the same species as those that eclosed from the same caterpillar morphospecies. However, if the individuals that eclosed from a single morphospecies belonged to multiple species, we re-evaluated the caterpillar morphology taking into account the caterpillar-adult matches. Individuals that could not be identified in this way were not assigned to any species (43 out of 237 in 2010 and 22 out of 203 in 2011). We also gathered information on host-plant specialization, flight period, winglessness in females and wingspan from the websites Catalogue of the Lepidoptera of Belgium (De Prins and Steeman 2021), and Guide to the moths of Great Britain and Ireland (Kimber 2021). We considered species that are monophagous oak feeders as specialists, and species that fly between June and October as having direct information of final leaf size while ovipositing.
Statistical analyses
To test for effects of phylogenetic isolation of individual host trees on proportional use of resources, we used multiple linear regression models. For folivores, we considered per-leaf folivory, per-individual folivory and per-leaf abundance as dependent variables that represent aspects of absolute leaf-resource use. These variables were then used in multiple regression models with a leaf trait (leaf area, LDMC, C:N, or phenolics), phylogenetic isolation, and the interaction between the leaf trait and phylogenetic isolation. Notably, in these models, the effects of leaf traits on leaf use represent the degree of proportional use of resources, while the interaction terms indicate the effects of phylogenetic isolation on proportional use of resources. For parasitoids, the dependent variable was the number of parasitised caterpillars, and predictors were the total number of caterpillars, phylogenetic isolation, and the interaction between the number of caterpillars and phylogenetic isolation. Tree pair was not included in the models because our analyses showed that resource use was not affected by pair (see Online Resource 2, Table S2). These analyses were repeated including crown position for the First Set of trees, and budburst phenology for the Second Set of trees as co-variables in the models (phenology had not been recorded for the First Set, and crown position had been held constant in the Second Set). Accounting for these covariables did not change the general conclusions, but reduced the degrees of freedom, and is hence presented only in Online Resource 3, Table S3. Finally, we repeated the initial models (the ones without the covariables), replacing phylogenetic isolation by either distance to the nearest oak, or by oak circumference (indicating age), as justified in the Introduction.
We note that per-leaf folivory cannot exceed the area of the leaf, and number of parasitised caterpillars cannot exceed the total number of caterpillars, so that a regression of, for instance, leaf area on folivory area is constrained to be positive. We hence did not present these particular relationships, nor the R2 of the total models, but our focus was on the interaction term, which is not affected by this constraint. Indeed, our hypotheses make predictions only about the interaction terms. We also note that multiple analyses were conducted on the effect of leaf quality, each accounting for different leaf characteristics. So, a correction for multiple testing might be warranted. However, even without correction we found only 2 significant interaction terms out of 21 in the main body of the manuscript (see Table 2) and hence conclude the absence of pattern, even without correction.
In ectophages, we explored whether proportional use of resources on phylogenetically non-isolated trees emerged in particular among species with certain traits. We therefore defined groups of species with a particular trait value, e.g., species that fly when the leaves are developed between June and October vs. other species. We then conducted for each of the two groups the initial model, i.e., per-leaf abundance explained by resource quantity or quality, and PI, and their interaction. We similarly divided the data into host-plant specialists and generalists, species with winged vs. wingless females, and species with below vs. above median wingspan.
To test for the contributions of different tree traits and leaf traits in shaping the species composition of ectophagous communities on individual trees, we performed a Permutational Multivariate Analysis of Variance (PERMANOVA) using the R package vegan (Oksanen et al. 2019). To test whether phylogenetic isolation sorts out certain species traits in ectophages, we used simple regressions: the dependent variable was the community weighted average value of the trait, using species abundance as a weight. In the calculus of community-weighted mean wingspan, we excluded species that have wingless females to ensure independence from the community-weighted mean of presence of wingless females. The predictor was the phylogenetic isolation of the trees.
All statistical analyses were performed using R version 4.0.2 (R Core Team 2020). We used a normal error distribution as the residuals of our multiple regression analyses approached normality and homoscedasticity. If a model did not meet normality and homoscedasticity criteria even after removal of at most two influential data points, it was removed from the analyses (3 out of 51 models in the body of the manuscript). Influential data points were identified by the visual assessment of four plots: residuals vs fitted values, normal Q-Q, scale-location, and residuals vs leverage (with Cook’s distance). We considered an interaction term significant only if it was significant after the removal of at most one influential data point and retained its significance after possible exclusion of a second influential data point.
Results
Overview of the data
Degree of phylogenetic isolation ranged from 5.71 to 106.67 million years in the First Set, and from 10 to 125.67 million years in the Second Set of trees, and varied continuously between the extremes (Online Resource 4, Fig. S4.a). Spatial distance from each study tree to the nearest adult oak ranged from 2.5 to 18.9 m in the First Set and from 0.7 to 9.4 m in the Second Set of trees (Online Resource 4, Fig. S4.b.). Circumference of the study trees ranged from 57.75 to 133.1 cm (mean 93.2 cm, SD = 22.4) in the First Set, and from 37.8 to 91.4 cm (mean 62.1 cm, SD = 16.7) in the Second Set of trees (Online Resource 4, Fig. S4.c).
Per tree mean leaf area ranged from 17.57 to 53.40 cm2 in the First Set of trees. In the Second Set, it ranged from 34.98 to 73.33 cm2 in 2010, and from 49.40 to 83.65 cm2 in 2011. Leaf size of a tree was positively correlated between 2010 and 2011 (t = 3.247, p = 0.004, df = 20). Mean LDMC ranged from 30.73 to 42.44% in the First Set of trees, and from 21.71 to 32.47% in the Second Set in 2010. Mean C:N ranged from 17.48 to 28.91 in the First Set, and from 9.41 to 13.65 in the Second Set in 2010. Mean leaf phenolics ranged from 3.04 to 10.01% in the First Set, and from 12.18 to 36.05% in the Second Set in 2010.
We found a total of 237 caterpillars on 9739 leaves in 2010, and 203 caterpillars on 14,914 leaves in 2011. The per-leaf abundance of caterpillars ranged from 0.004 to 0.072 in 2010, and from 0.003 to 0.028 in 2011. Out of these two years, the per-leaf abundance of caterpillars was higher in 2010 (Welch Two Sample t test t = 2.989, df = 28.203, p value = 0.006). The species composition of ectophage caterpillars on different trees is given in Online Resource 5, Table S5A. The information on wingspan, winglessness in females, flying period, host-plant specialization are given in Online Resource 5, Table S5B. Per-leaf abundance of leaf miners ranged from 0.050 to 0.610 in 2010, and from 0.200 to 0.800 in 2011. Per-leaf abundance of leaf gallers ranged from 0.003 to 0.362 in 2006, from 0.000 to 0.366 in 2010, and from 0.000 to 0.800 in 2011. For the caterpillars, per-leaf folivory ranged from 0.700 to 9.600 cm2 in the First Set of trees. In the Second Set of trees, it ranged from 0.525 to 9.525 cm2 in 2010, and from 0.425 to 10.600 cm2 in 2011. Per-individual folivory ranged from 21.197 to 418.5 cm2 in 2010, and from 39.462 to 803.480 cm2 in 2011. A total of 51 out of 206 caterpillars were parasitised in 2010 and 67 out of 203 in 2011.
Effect of phylogenetic isolation on proportional use of resources by ectophages
Phylogenetic isolation significantly reduced the proportional use of leaf size at all levels of leaf use: per-leaf folivory, per-individual folivory and per-leaf abundance (Fig. 2): the interaction term “leaf area × phylogenetic isolation” was significant and negative (Table 2). In particular, for per-leaf folivory this reduction was significant in all the study years i.e., for the First Set of trees in 2006 and for the Second Set in 2010 and in 2011 (Table 2, Fig. 2a). The levels of per-individual folivory and per-leaf abundance were tested only for the Second Set of trees in 2010 and in 2011. For per-individual folivory, the reduction was significant in 2011 but not in 2010 (Table 2, Fig. 2b). For per-leaf abundance (all species together), the reduction was significant in 2010 but not in 2011 (Table 2, Fig. 2c). In contrast, phylogenetic isolation did not significantly affect the proportional use of resource quality i.e., LDMC, C:N and phenolics (Table 2).
Phylogenetic isolation of individual host trees significantly reduced proportional use of leaf area by ectophages. a Phylogenetic isolation significantly reduced the increase in per-leaf folivory with an increase in leaf area in 2006 (interaction term phylogenetic isolation x leaf size: t = − 2.686, p = 0.019, df = 13), 2010 (t = − 3.078, p = 0.006, df = 18) and 2011 (t = − 2.122, p = 0.049, df = 17). b Phylogenetic isolation significantly reduced the increase in per-individual folivory with an increase in average leaf area in 2011 (t = − 2.329, p = 0.032, df = 17). (c) Phylogenetic isolation significantly reduced the increase in per-leaf abundance with an increase in leaf area in 2010 (t = − 2.844, p = 0.011, df = 18). Phylogenetic isolation is presented as binary in the figures, but is a continuous measure and statistically analysed as such
Within the different groups, we found that among specialists, phylogenetic isolation significantly reduced proportional use of resources in 2010 but significantly increased it in 2011 (Table 2, Fig. 3a). Among species with winged females, phylogenetic isolation significantly reduced proportional use of resources in 2010 but not in 2011 (Table 2).
Effect of phylogenetic isolation on subgroups of ectophages. a Phylogenetic isolation significantly reduced the increase in per-leaf abundance of specialists with an increase in leaf area in 2010 (t = − 2.377, p = 0.029, df = 18), but significantly increased the same in 2011 (t = 4.004, p < 0.001, df = 18). See Table 2 for full analyses. b Phylogenetic isolation significantly reduced the proportion of specialists in both 2010 (t = − 2.301, p = 0.033, df = 19) and 2011 (t = − 2.281, p = 0.034, df = 19). c Phylogenetic isolation significantly reduced the proportion of species that have wingless females in 2010 (t = − 2.535, p = 0.020, df = 19). See Table 3 for full analyses
In both the years 2010 and 2011, phylogenetic isolation contributed the most to shape the species composition in ectophage communities on individual trees, albeit only significantly in 2010 (Online Resource 6, Table S6). In particular, phylogenetic isolation significantly sorted out specialist ectophages in 2010 and 2011 (Table 3, Fig. 3b) and species with wingless females in 2010 (Table 3, Fig. 3c). An analysis based on the variable ‘presence/absence of species with wingless females’ using logistic regression leads to the same conclusion (Online Resource 8).
Effect of phylogenetic isolation on proportional use of resources by leaf gallers
Phylogenetic isolation did not significantly affect the proportional use of leaf size by leaf gallers. This effect was consistently absent in both the First Set (in 2006) and the Second Set (both in 2010 and in 2011) of trees (Table 2). Proportional use of leaf quality was affected significantly only in 1 out of 6 cases (Table 2). The interaction term “C:N × phylogenetic isolation” was positive (Table 2). This reflects a reduction in proportional use of low C:N (i.e., high leaf quality) with phylogenetic isolation.
Effect of phylogenetic isolation on proportional use of resources by leaf miners
The effect of phylogenetic isolation on proportional use of leaf resources by the leaf miners was inconsistent. For the Second Set of trees, proportional use of leaf size was significantly reduced in 2011 but not in 2010 (Table 2). Proportional use of leaf quality was affected significantly only in 1 out of 3 cases (Table 2): in terms of low C:N in the First Set of trees in 2010.
Effect of phylogenetic isolation on proportional use of resources by parasitoids
Phylogenetic isolation significantly reduced the proportional use of ectophagous caterpillars (total number of caterpillars) by parasitoids in 2010 but not in 2011 (Table 2 Fig. 4). We note that this decline in use of abundant caterpillars in 2010 cannot be explained by the effect of phylogenetic isolation on caterpillar diversity, because phylogenetic isolation reduced caterpillar diversity (p < 0.001, t = − 4.12, df = 20), and lower caterpillar diversity increased the number of parasitised caterpillars (p = 0.004, t = − 3.31, df = 19). We could not test for the effect of phylogenetic isolation on the proportional use of resource quality because we have no data on nutritional quality provided by the caterpillars.
Phylogenetic isolation of individual host trees significantly reduced proportional use of caterpillars by parasitoids in 2010. The increase in number of parasitised caterpillars with an increase in total number of caterpillars was less for phylogenetically isolated trees in 2010 (t = − 2.567, p = 0.02, df = 17), but not in 2011 (t = − 0.755, p = 0.460, df = 18). Note that the given statistics are for the interaction term ‘total number of caterpillars *phylogenetic isolation’. Phylogenetic isolation is presented as binary in the figures, but is a continuous measure and statistically analysed as such
Effect of isolation of resource patch in space and of age of the resource patch on proportional use of resources
Proportional use of resources was not affected consistently by isolation of resource patches in space, i.e. distance to nearest oak. Only 4 out of 36 models were significant – 1 for ectophages, 2 for leaf gallers, 1 for leaf miners and 0 for parasitoids (Online Resource 7A, Table S7A.a). The two effects on leaf gallers varied between years. In 2006, distance to nearest oak statistically reduced the proportional use of leaf size by leaf gallers, whereas in 2010 it reduced that of a quality parameter (low C:N). Proportional use of resources was never affected by the age of the resource patch, i.e. age of oaks measured by its circumference (Online Resource 7A, Table S7A.b). Overall, none of these variables affected proportional use of resources as consistently as did phylogenetic isolation. We finally note that the percentage of pines had a weaker effect than phylogenetic isolation (Online Resource 7B, Table S7B).
Discussion
We hypothesized that proportional use of resources by folivores and parasitoids would be reduced among more phylogenetically isolated trees as it reduces between-tree movement and masks search cues. We tested this hypothesis in three guilds of folivores—ectophages, leaf gallers, and leaf miners, and in parasitoids of ectophages. As predicted, we found that in ectophages the proportional use of leaf size was reduced on more phylogenetically isolated trees in terms of per-leaf folivory in all years tested. This pattern of per-leaf folivory appeared to be driven by per-individual folivory in 2011, and by per-leaf abundance in 2010. Patterns of per-leaf abundance in 2010, in turn, appeared to be driven by (1) an increase in abundances with leaf size on phylogenetically non-isolated trees in species that are specialists or have winged females in 2010, and (2) the decline of relative abundances of specialists and species with wingless-females on isolated trees in 2010. For leaf gallers and leaf miners, we did not find a consistent effect of phylogenetic isolation on proportional use of resources. For parasitoids of ectophages, phylogenetic isolation reduced the proportional use of caterpillars in one of the study years, and this cannot be explained by the finding that caterpillar diversity reduced parasitism. In contrast to the significant effects of phylogenetic isolation, we found no support for the further hypotheses of effects of spatial isolation from to the nearest oak or of tree age on proportional use of resources, and effects of phylogenetic isolation could not be explained simply by the proportion of pines.
Limitations
Our study inevitably has limitations. First, we studied the relationship between resource use and resource quantity or quality but did not directly study the processes that might drive resource use, such as movements or information usage by individuals. However, in comparable situations, such patterns have indeed been shown to result from movements or information usage. For instance, some studies showed that the usage of volatile compounds emitted by trees helps folivores to discriminate between host trees (Jactel et al. 2011; Ghirardo et al. 2012; Binyameen et al. 2013; Conchou et al. 2017). Moreover, we studied traits of ectophage species (for example, host-plant specialisation), which indirectly permits us to address the underlying mechanisms. Second, we interpret high folivory as a sign of high use of rich leaf resources—but it may also be a sign of compensatory feeding on poor-quality leaves (Raubenheimer and Simpson 1993). We cannot exclude a minor contribution of such compensatory feeding to our folivory scores, but consider a major contribution unlikely: compensatory feeding should decrease with leaf quality, but we found no negative relationship between folivory and leaf quality. Third, phylogenetic isolation might represent nothing more than an effect of the percentage of pine trees, the most abundant and phylogenetically most distant tree species in our study. However, replacing the phylogenetic isolation by % pines mostly reduced significances (Online Resource 7, Table 7B). Therefore, the observed effects of phylogenetic isolation on proportional use of resources are more than a pine-effect. Finally, folivory can also be due to folivores other than Lepidoptera caterpillars, notably certain Symphyta (Hymenoptera), Coleoptera and Orthoptera (Heil 2004; Martin et al. 2009). However, in this region, caterpillars are known to be by far the dominant ectophage folivores (personal observations and Southwood et al. 2004; Yguel et al. 2011). Moreover, Symphyta caterpillars were not observed.
How could phylogenetic isolation have reduced proportional use of leaf size by ectophages?
On phylogenetically isolated trees, folivory by ectophages did not increase proportionally to leaf size, in either of the study years. First, we had hypothesised that phylogenetic isolation of host trees might reduce the movement of specialized ectophages between neighbouring trees (Kennedy and Southwood 1984; Brändle and Brandl 2006; Gilbert and Webb 2007; Stratton et al. 2019). In this case, individual large-leaved trees would receive fewer ectophages from their neighbours when they are more phylogenetically isolated from their neighbours (suggested by Vialatte et al. 2010). This effect should be especially pronounced for more specialized folivore species (demonstrated for Heteroptera by Vialatte et al. 2010). As outlined above, declines of overall proportional use of resources in terms of per-leaf abundance were consistent with the patterns of absolute and relative abundance of specialists.
Second, the distantly related neighbours of phylogenetically isolated trees might mask the olfactory and visual cues of the focal tree (Binyameen et al. 2013), reducing information available to folivores. Hence, ectophages could find it more difficult to identify and reach large-leaved trees when the trees are more phylogenetically isolated (Jactel et al. 2011; Salazar et al. 2016). Furthermore, on small-leaved trees, the odour from the distantly related neighbours might discourage individual ectophages from leaving its host tree (Charnov 1976). Overall, when ectophages visit and test phylogenetically isolated trees at a lower rate or have less information about other potential host trees, they are less likely to show proportional use of resources. This should only play a role for folivores that oviposit when developed leaves are present, which could give them direct information about the size and quality of leaves. However, the effect of phylogenetic isolation on proportional use of resources did not differ between the species that have direct leaf size information during oviposition and species that do not have it.
Third, folivory by ectophages might not increase proportionally with leaf size on phylogenetically isolates trees due to poor sorting of phenotypes of ectophage species. If phylogenetic isolation of individual host trees reduces the influx of individual ectophages, it will also reduce the influx of phenotypes, which serve as the raw material for the sorting of those phenotypes that fit best to the local environment (a process operating both within and across species; Vellend 2016). With less raw material, phenotype sorting might be poor on more phylogenetically isolated trees. Hence, on large-leaved trees that are phylogenetically isolated, there might be fewer ectophages that are large and capable of eating much and thereby tracking large leaves. However, we did not find any effect of phylogenetic isolation on average body size (wingspan), nor did phylogenetic isolation affect the proportional use of leaf size by species with below or above median body size.
Fourth, abundances of ectophages may be abundant on some trees due to high in situ reproduction rather than immigration. In this scenario, larger leaves might allow larger populations to develop when the trees are phylogenetically non-isolated. This scenario would require that leaf size of a tree is correlated between years, which we found. The scenario would predict phylogenetic isolation of host trees to decrease relative abundances of species that are likely to reproduce in situ on their host tree: wingless-female species or oak specialists (being unable to use neighbouring non-oak trees). We indeed found decreases in relative abundances of these groups with phylogenetic isolation, in particular in 2010, the year in which ectophage abundances did not increase proportionally with leaf size on phylogenetically isolated trees. Moreover, the importance of in-situ reproduction is further underlined by the fact that even species in which ovipositing females do not have information on leaf size also showed proportional use of resources suggests an important role for in situ recruitment.
Overall, these four processes should facilitate using leaves proportional to their size on phylogenetically non-isolated trees. In theory we could have expected the opposite: that phylogenetically isolated trees harbour species that are very good at finding suitable trees and that may be able to use leaves proportional to their size even among phylogenetically isolated trees. Consistently, we did find more species with winged females on phylogenetically isolated trees. Nevertheless, leaves were used proportionally to their size only on the phylogenetically non-isolated trees. Altogether, that leaves are not used proportionally to their size on phylogenetically isolated trees cannot be explained by particular groups of ectophages becoming incapable of using leaves proportionally to their size, but possibly by a relative decline in those groups that might be best at using leaves proportionally to size.
Why was proportional use of resources achieved in terms of per-leaf abundance in one year, but by per-individual folivory in the other?
Patterns of proportional use of resources at the level of per-leaf folivory by ectophages were reflected in patterns of per-leaf abundance in 2010, and in patterns of per individual folivory in 2011. As outlined before, caterpillar abundance on non-isolated trees being proportional to leaf size suggests an establishment of little dispersive ectophages recruiting in situ on their host tree so that abundances on that tree correspond to the quantity of resources it offers. In contrast, patterns of per ectophage folivory suggest sorting of ectophage phenotypes by the leaf traits. Thus, within ectophage species, trees with larger leaves would accumulate ectophage genotypes that consume more leaf surface, or across ectophage species, those species that consume more leaf surface. The importance of these two groups of processes may depend on the overall abundance of ectophages, which was significantly higher in 2010 than in 2011. High abundance might trigger negative density-dependent interactions among ectophages such as direct resource competition, increased defences of the shared host, or attraction of shared natural enemies (Birkett et al. 2003; Staudt and Lhoutellier 2007; Faiola and Taipale 2020; Collie et al. 2020). These negative density-dependent interactions, in turn, may push ectophages to leave host trees, and find new host trees (Charnov 1976; Vialatte et al. 2010). Phylogenetic isolation may then hamper these processes, in particular in a high-abundance year like 2010. The same negative density-dependent processes among abundant ectophages also reduces the amount of resources available per ectophage (Collie et al. 2020). As a result, in a high abundance year, increased leaf area per tree may not correspond to increased leaf area available per ectophage, and hence not sort for ectophage phenotypes that are capable of eating more. Overall, the high total abundance of ectophages in 2010 might possibly explain why ectophage abundance, but not per ectophage folivory increased with leaf area on non-isolated trees (and inversely for 2011).
Why did phylogenetic isolation not affect the ectophage’s proportional use of leaf quality?
We suggest that proportional use of leaf quality by ectophages was not affected by phylogenetic isolation because there was no proportional use of leaf quality. When proportional use of leaf quality is absent, it cannot be affected by phylogenetic isolation. Proportional use of leaf quality may be absent when information on leaf quality is insufficient. This is most obvious for folivores that oviposit during the winter when there are no leaves in the trees (Sarvašová et al. 2020). Even folivores that oviposit in late summer and overwinter as eggs to hatch in spring (Du Merle 1988) may face a poor correlation between leaf quality in summer and that in spring of the next year. Even in the same season, leaf quality does not correlate perfectly among years (Gripenberg et al. 2007). Such poor correlation might be caused in part by the tree’s responses to folivory: trees are known to respond to folivory by reducing their leaf quality for folivores (Kant et al. 2015; Volf et al. 2021). Therefore, herbivorous adult insects not emerging in spring might lack sufficient information on quality of leaves available to larvae in spring. This problem might be particularly strong in trees that suffer much from folivory, which are often phylogenetically non-isolated trees (Yguel et al. 2011). For this reason, resource quality might not be tracked even on non-isolated trees.
Why did phylogenetic isolation not reduce proportional use of resources by leaf gallers and leaf miners?
Proportional use of resources by leaf gallers and leaf miners was not consistently affected by phylogenetic isolation of individual host trees. This is consistent with lack of effects of phylogenetic isolation on overall abundance of leaf miners and leaf gallers in the study system (Hidasi-Neto et al. 2018). Perhaps miners and gallers do not respond to the parameters tested. Absence of proportional use of leaf size was surprising, given reported preferences for large leaves in leaf miners (Faeth 1991). Endophages might have little to no need of choosing trees with a particular leaf quality, because they can strongly improve it locally (Cornell 1989; Hartley 1998; Nyman and Julkunen-Tiitto 2000; Giron et al. 2007; Kaiser et al. 2010). Perhaps other leaf traits such as toughness that limit oviposition and mine initiation are more important to endophages (Faeth 1985; Pihain et al. 2019). Even when endophages do track resources, it might be easier for them to overcome the effect of phylogenetic isolation than it is for ectophages if they i.e. experience less dispersal limitation. Specifically, many leaf gallers are known for their high dispersal capacity (Gilioli et al. 2013). In addition, this high dispersal limits in situ reproduction on host trees and therefore adjustment of abundances to resources available on a tree (Connor et al. 1983).
Why did parasitoids use hosts proportionally in only one of the two study years?
The proportional use of ectophagous caterpillars by parasitoids was reduced by phylogenetic isolation of individual host trees in 2010, but not in 2011. This pattern of 2010 cannot be explained by high caterpillar diversity possibly reducing parasitism (Stireman III and Singer 2003), as we have demonstrated that phylogenetic isolation reduced caterpillar diversity and would thus have increased parasitism on phylogenetically isolated trees, not decreased it. One possible explanation could be the higher abundance of ectophagous caterpillars in 2010 (Yguel et al. 2014). Effect of abundance may be predicted from optimal foraging theory: many parasitoid species exhibit a type III functional response to host density (Morrison and Strong 1980; Fernández-arhex and Corley 2003; Veldtman and McGeoch 2004), which means that resource use increases with resource abundance only above a certain threshold of resource abundance. In our case, such high levels of abundances of ectophages might have been reached only in the high abundance year, and even then, only on the phylogenetically non-isolated trees. Our data thus suggest that parasitoids track the abundance of ectophage hosts only during years of high overall ectophage abundance and among trees with high ectophage abundance (phylogenetically non-isolated). We finally note that parasitism not only increases in a uniformly closely related tree neighbourhood, it also increases in a uniform community of host caterpillars.
What are the potential consequences for trees that are phylogenetically isolated?
The fact that ectophagy mostly does not increase with leaf area on phylogenetically isolated trees, may be both advantageous and disadvantageous for the trees. On the one hand, it may be advantageous because when folivores cannot feed more on large-leaved trees, trees might then benefit more from large leaves. Such leaves can help to reduce water loss (Wang et al. 2019). Hence, phylogenetically isolated trees can be expected to grow larger leaves. Consistently, Castagneyrol et al. (2017) found that specific leaf area increases with phylogenetic isolation of individual host trees. On the other hand, the fact that ectophagy mostly does not increase with leaf area on phylogenetically isolated trees may also be disadvantageous for the trees, as it renders attacks by ectophages unpredictable. Unpredictable attacks select for induced defences because constitutive defence would be a waste of resources when there is no attack. However, when there is an attack, induced defences may be costlier than constitutive defences (Pigliucci 2001; Perkovich and Ward 2021). Overall, for a tree, the advantages and disadvantages of growing in a phylogenetically distant neighbourhood might possibly equal out, but the selection pressures on leaf area and defence traits are likely to be different.
Conclusions
Overall, we find that proportional use of resources declines with phylogenetic isolation of host trees. It does so in the folivore guild that is least capable of moving between, detecting and manipulating host trees — the ectophages. Ectophagous folivores consume more on large-leaved trees, either by establishing in larger numbers of individuals (2010), or by establishing individual phenotypes that consume more (2011). But this proportional use of resources happens only as long as neighbouring trees are phylogenetically proximate, in 2010 likely because in such neighbourhoods species accumulate that are most likely to recruit locally — species specialized on oaks or having wingless females. Parasitism could partly counteract proportional use of resources on trees in phylogenetically proximate neighbourhoods, because high densities of ectophages attract more parasitoids, at least during high-abundance years. Lack of proportional use of resources in leaf miners and leaf gallers might be due to their ability to improve resource quality within trees, and due to their limited in situ recruitment on trees. Finally, the negative effect of phylogenetic isolation of trees on proportional use of resources by ectophages suggests that tree populations in phylogenetically distant neighbourhoods might be selected for larger leaves and greater reliance on induced defences.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The R-codes used for the data analyses during the current study are available from the corresponding author on reasonable request.
References
Aartsma Y, Bianchi FJJA, van der Werf W et al (2017) Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol 216:1054–1063. https://doi.org/10.1111/nph.14475
Asplen MK (2018) Dispersal strategies in terrestrial insects. Curr Opin Insect Sci 27:16–20. https://doi.org/10.1016/j.cois.2018.01.009
Avgar T, Betini GS, Fryxell JM (2020) Habitat selection patterns are density dependent under the ideal free distribution. J Anim Ecol 89:2777–2787. https://doi.org/10.1111/1365-2656.13352
Bailey R, Schönrogge K, Cook JM et al (2009) Host niches and defensive extended phenotypes structure parasitoid wasp communities. PLOS Biol 7:e1000179. https://doi.org/10.1371/journal.pbio.1000179
Barker HL, Holeski LM, Lindroth RL (2018) Genotypic variation in plant traits shapes herbivorous insect and ant communities on a foundation tree species. PLoS ONE 13:e0200954. https://doi.org/10.1371/journal.pone.0200954
Bernays EA, Funk DJ (1999) Specialists make faster decisions than generalists: experiments with aphids. Proc R Soc Lond B Biol Sci 266:151–156. https://doi.org/10.1098/rspb.1999.0615
Binyameen M, Hussain A, Yousefi F et al (2013) Modulation of reproductive behaviors by non-host volatiles in the Polyphagous Egyptian cotton leafworm, Spodoptera littoralis. J Chem Ecol 39:1273–1283
Birkett MA, Chamberlain K, Guerrieri E et al (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29:1589–1600. https://doi.org/10.1023/A:1024218729423
Brändle M, Brandl R (2006) Is the composition of phytophagous insects and parasitic fungi among trees predictable? Oikos 113:296–304. https://doi.org/10.1111/j.2006.0030-1299.14418.x
Castagneyrol B, Giffard B, Péré C, Jactel H (2013) Plant apparency, an overlooked driver of associational resistance to insect herbivory. J Ecol 101:418–429. https://doi.org/10.1111/1365-2745.12055
Castagneyrol B, Bonal D, Damien M et al (2017) Bottom-up and top-down effects of tree species diversity on leaf insect herbivory. Ecol Evol 7:3520–3531. https://doi.org/10.1002/ece3.2950
Charlery de la Masselière M, Facon B, Hafsi A, Duyck P-F (2017) Diet breadth modulates preference performance relationships in a phytophagous insect community. Sci Rep 7:16934. https://doi.org/10.1038/s41598-017-17231-2
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136. https://doi.org/10.1016/0040-5809(76)90040-x
Collie J, Granela O, Brown EB, Keene AC (2020) Aggression is induced by resource limitation in the Monarch caterpillar. iScience 23:101791. https://doi.org/10.1016/j.isci.2020.101791
Conchou L, Anderson P, Birgersson G (2017) Host plant species differentiation in a polyphagous moth: olfaction is enough. J Chem Ecol 43:794–805. https://doi.org/10.1007/s10886-017-0876-2
Connor EF, Faeth SH, Simberloff D (1983) Leafminers on oak: the role of immigration and in situ reproductive recruitment. Ecology 64:191–204. https://doi.org/10.2307/1937340
Corff JL, Marquis RJ (1999) Differences between understorey and canopy in herbivore community composition and leaf quality for two oak species in Missouri. Ecol Entomol 24:46–58. https://doi.org/10.1046/j.1365-2311.1999.00174.x
Cornelissen JHC, Lavorel S, Garnier E et al (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335. https://doi.org/10.1071/BT02124
Cornelissen T, Stiling P (2008) Clumped distribution of oak leaf miners between and within plants. Basic Appl Ecol 9:67–77. https://doi.org/10.1016/j.baae.2006.08.007
Cornell HV (1989) Endophage-ectophage ratios and plant defense. Evol Ecol 3:64–76. https://doi.org/10.1007/BF02147932
Courtney SP, Kibota TT (1990) Mother Doesn’t Know Best: Selection of Hosts by Ovipositing Insects. Taylor & Francis Group, Boca Raton
Craig TP, Itami JK (2008) Evolution of preference and performance relationships. University of California Press
Crawley MJ, Akhteruzzaman M (1988) Individual variation in the phenology of oak trees and its consequences for herbivorous insects. Funct Ecol 2:409–415. https://doi.org/10.2307/2389414
De Moraes CM, Lewis WJ, Paré PW et al (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573. https://doi.org/10.1038/31219
De Prins W, Steeman C (2021) Catalogue of the Lepidoptera of Belgium. https://projects.biodiversity.be/lepidoptera/. Accessed 24 Feb 2022
Du Merle P (1988) Phenological resistance of oaks to the green oak leafroller, Tortrix viridana (Lepidoptera: Tortricidae). In: Mattson WJ, Levieux J, Bernard-Dagan C (eds) Mechanisms of Woody Plant Defenses Against Insects. Springer, New York, NY, pp 215–226
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608. https://doi.org/10.2307/2406212
Eisenring M, Unsicker SB, Lindroth RL (2021) Spatial, genetic and biotic factors shape within-crown leaf trait variation and herbivore performance in a foundation tree species. Funct Ecol 35:54–66. https://doi.org/10.1111/1365-2435.13699
Ekholm A, Tack AJM, Pulkkinen P, Roslin T (2020) Host plant phenology, insect outbreaks and herbivore communities – The importance of timing. J Anim Ecol 89:829–841. https://doi.org/10.1111/1365-2656.13151
Eliason EA, Potter DA (2000) Impact of whole-canopy and systemic insecticidal treatments on Callirhytis cornigera (Hymenoptera: Cynipidae) and associated parasitoids on pin oak. J Econ Entomol 93:165–171. https://doi.org/10.1603/0022-0493-93.1.165
Emlen JM (1966) The role of time and energy in food preference. Am Nat. https://doi.org/10.1086/282455
Faeth SH (1985) Quantitative defense theory and patterns of feeding by oak insects. Oecologia 68:34–40. https://doi.org/10.1007/BF00379470
Faeth SH (1991) Effect of oak leaf size on abundance, dispersion, and survival of the leafminer Cameraria sp. (Lepidoptera: Gracillariidae). Environ Entomol 20:196–204. https://doi.org/10.1093/ee/20.1.196
Faeth SH, Mopper S, Simberloff D (1981) Abundances and diversity of leaf-mining insects on three oak host species: effects of host-plant phenology and nitrogen content of leaves. Oikos 37:238–251. https://doi.org/10.2307/3544471
Faiola C, Taipale D (2020) Impact of insect herbivory on plant stress volatile emissions from trees: a synthesis of quantitative measurements and recommendations for future research. Atmospheric Environ X 5:100060. https://doi.org/10.1016/j.aeaoa.2019.100060
Faticov M, Ekholm A, Roslin T, Tack AJM (2020) Climate and host genotype jointly shape tree phenology, disease levels and insect attacks. Oikos 129:391–401. https://doi.org/10.1111/oik.06707
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581. https://doi.org/10.2307/1934037
Fernández-arhex V, Corley JC (2003) The functional response of parasitoids and its implications for biological control. Biocontrol Sci Technol 13:403–413. https://doi.org/10.1080/0958315031000104523
Forister ML, Novotny V, Panorska AK et al (2015) The global distribution of diet breadth in insect herbivores. Proc Natl Acad Sci 112:442–447. https://doi.org/10.1073/pnas.1423042112
Forkner RE, Marquis RJ, Lill JT (2004) Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol Entomol 29:174–187. https://doi.org/10.1111/j.1365-2311.2004.0590.x
Fretwell SD, Lucas HL (1969) On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor 19:16–36. https://doi.org/10.1007/BF01601953
Ghirardo A, Heller W, Fladung M et al (2012) Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana: Detrimental effect of HIPV emissions in oaks. Plant Cell Environ 35:2192–2207. https://doi.org/10.1111/j.1365-3040.2012.02545.x
Gilbert G, Webb C (2007) Phylogenetic signal in plant pathogen–host range. Proc Natl Acad Sci U S A 104:4979–4983. https://doi.org/10.1073/pnas.0607968104
Gilioli G, Pasquali S, Tramontini S, Riolo F (2013) Modelling local and long-distance dispersal of invasive chestnut gall wasp in Europe. Ecol Model 263:281–290. https://doi.org/10.1016/j.ecolmodel.2013.05.011
Giron D, Kaiser W, Imbault N, Casas J (2007) Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol Lett 3:340–343. https://doi.org/10.1098/rsbl.2007.0051
Gossner M, Chao A, Bailey R, Prinzing A (2009) Native fauna on exotic trees: phylogenetic conservatism and geographic contingency in two lineages of phytophages on two lineages of trees. Am Nat 173:599–614. https://doi.org/10.1086/597603
Gossner MM, Weisser WW, Gershenzon J, Unsicker SB (2014) Insect attraction to herbivore-induced beech volatiles under different forest management regimes. Oecologia 176:569–580. https://doi.org/10.1007/s00442-014-3025-4
Gripenberg S, Morriën E, Cudmore A et al (2007) Resource selection by female moths in a heterogeneous environment: what is a poor girl to do? J Anim Ecol 76:854–865. https://doi.org/10.1111/j.1365-2656.2007.01261.x
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference–performance relationships in phytophagous insects. Ecol Lett 13:383–393. https://doi.org/10.1111/j.1461-0248.2009.01433.x
Hartley SE (1998) The chemical composition of plant galls: are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 113:492–501. https://doi.org/10.1007/s004420050401
Hartley SE, Lawton JH (1992) Host-plant manipulation by gall-insects: a test of the nutrition hypothesis. J Anim Ecol 61:113–119. https://doi.org/10.2307/5514
Heil M (2004) Direct defense or ecological costs: responses of herbivorous beetles to volatiles released by wild lima bean (Phaseolus lunatus). J Chem Ecol 30:1289–1295. https://doi.org/10.1023/B:JOEC.0000030299.59863.69
Hidasi-Neto J, Bailey RI, Vasseur C et al (2018) A forest canopy as a living archipelago: why phylogenetic isolation may increase and age decrease diversity. J Biogeogr 46:158–169. https://doi.org/10.1111/jbi.13469
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320. https://doi.org/10.4039/Ent91293-5
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top- down forces in natural communities. Ecology 73:724–732
Jactel H, Birgersson G, Andersson S, Schlyter F (2011) Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 166:703–711. https://doi.org/10.1007/s00442-011-1918-z
Javoiš J, Davis RB, Tammaru T (2019) A comparative morphometric study of sensory capacity in geometrid moths. J Evol Biol 32:380–389. https://doi.org/10.1111/jeb.13422
Kaiser W, Huguet E, Casas J et al (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc R Soc B Biol Sci 277:2311–2319. https://doi.org/10.1098/rspb.2010.0214
Kant MR, Jonckheere W, Knegt B et al (2015) Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann Bot 115:1015–1051. https://doi.org/10.1093/aob/mcv054
Kennedy CEJ, Southwood TRE (1984) The number of species of insects associated with British trees: a re-analysis. J Anim Ecol 53:455–478. https://doi.org/10.2307/4528
Kimber I (2021) UKMoths | Guide to the moths of Great Britain and Ireland. https://ukmoths.org.uk/. Accessed 24 Feb 2022
Kitamura M, Nakamura T, Hattori K et al (2007) Among-tree variation in leaf traits and herbivore attacks in a deciduous oak, Quercus dentata. Scand J for Res 22:211–218. https://doi.org/10.1080/02827580701217893
Kratina P, Vos M, Bateman A, Anholt BR (2009) Functional responses modified by predator density. Oecologia 159:425–433. https://doi.org/10.1007/s00442-008-1225-5
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Martin CG, Mannion C, Schaffer B (2009) Effects of herbivory by Diaprepes abbreviatus (Coleoptera: Curculionidae) larvae on four woody ornamental plant species. J Econ Entomol 102:1141–1150. https://doi.org/10.1603/029.102.0336
Moore R, Francis BJ (1991) Factors influencing herbivory by insects on oak trees in pure stands and paired mixtures. J Appl Ecol 28:305. https://doi.org/10.2307/2404131
Morrison G, Strong D (1980) Spatial variations in host density and the intensity of parasitism: some empirical examples. Environ Entomol 9(2):l49–152. https://doi.org/10.1093/EE/9.2.149
Novotny V, Miller SE, Baje L et al (2010) Guild-specific patterns of species richness and host specialization in plant-herbivore food webs from a tropical forest. J Anim Ecol 79:1193–1203
Nyman T, Julkunen-Tiitto R (2000) Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc Natl Acad Sci 97:13184–13187. https://doi.org/10.1073/pnas.230294097
Oksanen J, Guillaume Blanchet F, Friendly M et al (2019) vegan: Community Ecology Package. Version 2.5–6.URL https://CRAN.R-project.org/package=vegan. Accessed 17 Mar 2022
Perfecto I, Vet L (2003) Effect of a nonhost plant on the location behaviour of two parasitoids: the tritrophic system of Cotesia spp. (Hymenoptera: Braconidae), Pieris rapae (Lepidoptera: Pieridae), and Brassica oleraceae. Environ Entomol - Env ENTOMOL 32:163–174. https://doi.org/10.1603/0046-225X-32.1.163
Perkovich C, Ward D (2021) Herbivore-induced defenses are not under phylogenetic constraints in the genus Quercus (oak): Phylogenetic patterns of growth, defense, and storage. Ecol Evol 11:5187–5203. https://doi.org/10.1002/ece3.7409
Peterson MA, Denno RF (1998) The influence of dispersal and diet breadth on patterns of genetic isolation by distance in phytophagous insects. Am Nat 152:428–446. https://doi.org/10.1086/286180
Pigliucci M (2001) Phenotypic Plasticity. In: Fox CW, Roff DA, Fairbairn DJ (eds) Evolutionary Ecology: Concepts and Case Studies. Oxford University Press, Oxford, New York, pp 58–69
Pihain M, Gerhold P, Ducousso A, Prinzing A (2019) Evolutionary response to coexistence with close relatives: increased resistance against specialist herbivores without cost for climatic-stress resistance. Ecol Lett 22:1285–1296. https://doi.org/10.1111/ele.13285
Pilosof S, Porter MA, Pascual M, Kéfi S (2017) The multilayer nature of ecological networks. Nat Ecol Evol 1:1–9. https://doi.org/10.1038/s41559-017-0101
R Core Team (2020) R: a language and environment for statistical computing. https://www.r-project.org/. Accessed 20 Dec 2020
Raubenheimer D, Simpson SJ (1993) The geometry of compensatory feeding in the locust. Anim Behav 45:953–964. https://doi.org/10.1006/anbe.1993.1114
Rozas V (2003) Tree age estimates in Fagus sylvatica and Quercus robur: testing previous and improved methods. Plant Ecol 167:193–212. https://doi.org/10.1023/A:1023969822044
Ruhnke H, Schädler M, Klotz S et al (2009) Variability in leaf traits, insect herbivory and herbivore performance within and among individuals of four broad-leaved tree species. Basic Appl Ecol 10:726–736. https://doi.org/10.1016/j.baae.2009.06.006
Salazar D, Jaramillo A, Marquis RJ (2016) The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 181:1199–1208. https://doi.org/10.1007/s00442-016-3629-y
Sarvašová L, Kulfan J, Saniga M et al (2020) Winter geometrid moths in oak forests: Is monitoring a single species reliable to predict defoliation risk? Forests 11:288. https://doi.org/10.3390/f11030288
Schär S, Vorburger C (2013) Host specialization of parasitoids and their hyperparasitoids on a pair of syntopic aphid species. Bull Entomol Res 103:1–8. https://doi.org/10.1017/S0007485313000114
Schoonhoven LM, Loon BV, van Loon JJA, Dicke M (2005) Insect-Plant Biology. OUP, Oxford
Seifert CL, Volf M, Jorge LR et al (2020) Plant phylogeny drives arboreal caterpillar assemblages across the Holarctic. Ecol Evol 10:14137–14151. https://doi.org/10.1002/ece3.7005
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Southwood TRE, Wint GRW, Kennedy CEJ, Greenwood SR (2004) Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. Eur J Entomol 101:43–50. https://doi.org/10.14411/eje.2004.011
Staudt M, Lhoutellier L (2007) Volatile organic compound emission from holm oak infested by gypsy moth larvae: evidence for distinct responses in damaged and undamaged leaves. Tree Physiol 27:1433–1440. https://doi.org/10.1093/treephys/27.10.1433
Stireman JO III, Singer MS (2003) Determinants of parasitoid–host associations: insights from a natural Tachinid-Lepidopteran community. Ecology 84:296–310. https://doi.org/10.1890/0012-9658(2003)084[0296:DOPHAI]2.0.CO;2
Stratton CA, Hodgdon E, Rodriguez-Saona C et al (2019) Odors from phylogenetically-distant plants to Brassicaceae repel an herbivorous Brassica specialist. Sci Rep 9:10621. https://doi.org/10.1038/s41598-019-47094-8
van Beest FM, McLoughlin PD, Mysterud A, Brook RK (2016) Functional responses in habitat selection are density dependent in a large herbivore. Ecography 39:515–523. https://doi.org/10.1111/ecog.01339
Veldtman R, McGeoch MA (2004) Spatially explicit analyses unveil density dependence. Proc R Soc Lond B Biol Sci 271:2439–2444. https://doi.org/10.1098/rspb.2004.2905
Vellend M (2016) The Theory of Ecological Communities (MPB-57). Princeton University Press
Vialatte A, Bailey RI, Vasseur C et al (2010) Phylogenetic isolation of host trees affects assembly of local Heteroptera communities. Proc R Soc B Biol Sci 277:2227–2236. https://doi.org/10.1098/rspb.2010.0365
Volf M, Volfová T, Seifert CL et al (2021) A mosaic of induced and non-induced branches promotes variation in leaf traits, predation and insect herbivore assemblages in canopy trees. Ecol Lett n/a: https://doi.org/10.1111/ele.13943
Wang C, He J, Zhao T-H et al (2019) The smaller the leaf is, the faster the leaf water loses in a temperate forest. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00058
Wesołowski T, Rowiński P (2006) Timing of bud burst and tree-leaf development in a multispecies temperate forest. For Ecol Manag 237:387–393. https://doi.org/10.1016/j.foreco.2006.09.061
West C (1985) Factors underlying the late seasonal appearance of the lepidopterous leaf-mining guild on oak. Ecol Entomol 10:111–120. https://doi.org/10.1111/j.1365-2311.1985.tb00540.x
Wimp GM, Young WP, Woolbright SA et al (2004) Conserving plant genetic diversity for dependent animal communities. Ecol Lett 7:776–780. https://doi.org/10.1111/j.1461-0248.2004.00635.x
Yguel B, Bailey R, Tosh N et al (2011) Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecol Lett 14:1117–1124. https://doi.org/10.1111/j.1461-0248.2011.01680.x
Yguel B, Bailey RI, Villemant C et al (2014) Insect herbivores should follow plants escaping their relatives. Oecologia 176:521–532. https://doi.org/10.1007/s00442-014-3026-3
Acknowledgements
We thank Guillaume Gauthier from LiberTree for the climbing training, Cynthia Gauthier, Amaury Brault and Elisa Grégoire for their help in sampling and rearing, the Office National des Forêts for logistic support, and Christa Schaffellner’s team for support in the 2006’s chemical analyses. We also thank Mickael Pihain and Loréne Julia Marchand for fruitful discussions and insightful comments. This work is supported by an ACOMB grant from the Région Bretagne, an ATIP grant from CNRS. BY was supported by a doctoral grant from Région Bretagne and CNRS, and SM was supported by a doctoral grant from the Ministry of Research and Education (France), a travel grant from doctoral school EGAAL, and a mobility grant from Region de Bretange.
Funding
This study was funded by an ACOMB grant from the Région Bretagne, an ATIP grant from CNRS. BY was supported by a doctoral grant from Région Bretagne and CNRS, and SM was supported by a doctoral grant from the Ministry of Research and Education (France).
Author information
Authors and Affiliations
Contributions
SM, AP, FM, and JM: conceptualized the study and developed it. BY, RB and FJ: produced the data. SM: analysed the data. SM, FM and AP: drafted the initial manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Consent of participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Riccardo Bommarco.
Trees surrounded by phylogenetically distant neighbours are little used by specialist leaf-chewers. Thus, such trees can have large leaves without suffering extensive leaf damage.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mallick, S., Molleman, F., Yguel, B. et al. Ectophagous folivores do not profit from rich resources on phylogenetically isolated trees. Oecologia 201, 1–18 (2023). https://doi.org/10.1007/s00442-022-05260-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05260-2