Abstract
Soil microbial communities can have an important role in the adaptation of plants to their local abiotic soil conditions and in mediating plant responses to environmental stress. This has been clearly demonstrated for individual plant species, but it is unknown how locally adapted microbes may affect plant communities. It is possible that the adaptation of microbial communities to local conditions can shape plant community composition. Additionally, it is possible that the effects of locally adapted microorganisms on individual plant species could be altered by co-occurring plant species. We tested these possibilities in plant community mesocosms with soils and mycorrhizal fungi (AMF) from three locations. We found that plant community biomass responded positively to local adaptation of AMF to soil conditions. Plant community composition also changed in response to local adaptation of AMF. Unexpectedly, the strongest benefits of locally adapted AMF went to early successional plant species that have the highest relative growth rates and the lowest responsiveness to the presence of AMF. Late successional plants that responded positively overall to the presence of AMF were often suppressed in communities with local AMF, perhaps because of strong competition from fast growing plant species. These results show that local adaptation of soil microbial communities can shape plant community composition, and the benefits that plants derive from locally adapted microorganisms can be reshaped by the competitive context in which these associations occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil microbial communities can determine the relative abundance of plant species and the overall productivity of plant communities (Reynolds et al. 2003). Additionally, soil microorganisms are important for plant adaptation to local soil conditions and mediating plant responses to environmental stress (Johnson et al. 2010; Barrett et al. 2012; Lau and Lennon 2012; Delavaux et al. 2017). Overall, tests of microbial effects on plant communities and on local adaptation are typically considered separately, with tests of microbial mediation of plant adaptation limited to tests of single plant species (e.g. Schultz et al. 2001; Johnson et al. 2010; Sherrard and Maherali 2012). However, because plant species vary in their responses to soil microbial communities (van der Heijden et al. 1998; Wilson and Hartnett 1998), it is likely that adaptation of microorganisms to local soil conditions has important consequences for the effects of microorganisms on plant community composition and productivity.
Arbuscular mycorrhizal fungi (AMF), in particular, can be important mediators of local adaptation (Rúa et al. 2016). For example, AMF have an important role in local adaptation of Andropogon gerardii, a dominant tallgrass prairie plant, to soil nutrient levels (Schultz et al. 2001; Johnson et al. 2010). Nutrient uptake is an important benefit that plants gain from AMF, however, association with mycorrhizae is associated with other benefits, including drought tolerance (Delavaux et al. 2017). It is possible that in addition to adaptation to soil nutrients, mycorrhizae could play a key role in adaptation of plants to local soil moisture conditions (Stahl and Smith 1984). However, we do not know the relative extent to which soils or climate are important factors in mycorrhizal mediation of plant adaptation.
AMF can also shift the relative abundance of plant species within plant communities, favoring the species that are most reliant on this mutualism (Hartnett and Wilson 1999; Bauer et al. 2012; Middleton and Bever 2012). If the benefits that AMF deliver to plant species depend on local adaptation of the fungi, then we should expect that AMF responsive plant species will increase in abundance when associated with locally adapted AMF. However, we do not know the potential for local adaptation to cause changes in species composition because tests of microbe-mediated local adaptation have been limited to experiments on single plant species. In our study system, AMF responsiveness is associated with life-history trade-offs, and late-successional plant species show greater responsiveness (Koziol and Bever 2015, 2017; Bauer et al. 2018) and specificity of response to AMF (Koziol and Bever 2016). We would therefore expect that late successional plant species would benefit most from adaptation of AMF to local soil conditions.
In this paper, we test for plant community responses to local adaptation of mycorrhizae to local soil and drought conditions. We hypothesized that mycorrhizae would mitigate the negative effects of drought on plants, and we expected that mycorrhizae originating from more drought-prone environments would deliver greater benefits to plants under drought conditions compared to fungi from more mesic sites. We further test if local adaptation of fungi to soil conditions results in greater benefits to their plant hosts. We conduct these tests using mixtures of individual cultures of local mycorrhizae as inocula, thereby allowing for the mycorrhizal mediation of plant adaptation to be mediated by effects of individual fungal isolates or the effects of consortium. We also conduct these tests on diverse plant communities, including both low mycorrhizal responsive, early successional and highly mycorrhizal responsive, late successional plant species, allowing us to test context dependence of mycorrhizal mediation of local adaptation across successional stage and the effects of mycorrhizal fungi on plant community composition. We predicted that local adaption of AMF would provide the strongest benefits to late-successional, mycorrhizae dependent plant species. As we are specifically focused on consequences of adaptation of AMF to soil and climate, we use non-locally adapted plant hosts from an intermediate location to control for possible effects of plant adaptation to soils, climate or AMF.
Methods
Study system
Our study was based in the tallgrass prairies of the Midwestern United States, including study sites in Illinois, Kansas, and Oklahoma. There is significant variation in climate between these study sites with less precipitation and greater rates of evapotranspiration at our study sites in Kansas and Oklahoma as compared to Illinois (Sanford and Selnick 2013). From these three sites we isolated mycorrhizal fungi from soils collected from remnant tallgrass prairies. We then established mesocosms in 7.5L pots (20 cm width × 32 cm height, Treepots, Stewe and Sons, Tangent, Oregon) and filled these pots with sterilized soil from each of our study sites. Each mesocosm was inoculated with fungi from one of our study sites or retained as a sterile control in a 3 × 4 design. These mesocosms were then planted with nine species of tallgrass prairie plants common to each of the study regions. Half of our mesocosms were watered to near field capacity and the remaining received half as much water, corresponding to typical drought conditions in our study area. In total, each soil × fungi × drought treatment included 7–10 replicates (with some replication limited by availability of soil or fungal inoculum), and our experiment included 199 total mesocosms (three soil treatments × four fungi treatments × two drought treatments × 7–10 replicates = 199 mesocosms). Our experiment was arranged on greenhouse benches in 10 blocks containing one replicate of each treatment.
Soil treatments
We collected soil from each of our study sites from areas near the remnant prairies from which we isolated our mycorrhizae cultures. Soil collected from Illinois was Dana Silt Loam, Kansas was Reading Silt Loam, and Oklahoma was Grainola Clay-Loam. Before use in the experiment, we mixed all soils 1:1 with sand to facilitate drainage under greenhouse conditions. Then, we pasteurized the three different background soils with an electric soil sterilizer (Model SS-60; Pro-Grow Supply, Brookfield, WI, USA) at 190°C for 4 h, rested the soil for 24 h, and pasteurized again for 4 h. This sterilization technique effectively controls AMF, with AMF abundance eliminated or reduced by over 90% relative to controls at the conclusion of past experiments with similar experimental designs (Koziol and Bever 2015, 2016).
AM Fungal treatments
AMF were isolated from remnant prairie soils collected from Illinois, Kansas, and Oklahoma (Electronic Resource, Table 1). We created fungal cultures by sorting spores microscopically by morphotype. Morphotypes were grown with Sorghum bicolor host plants for 6 months in their relevant sterilized background soils. To initiate this experiment single species fungal cultures were mixed together to create diverse fungal mixtures relevant to each of the three sites. As our goal was to best represent each AMF community, we used a single isolate from each AMF species isolated from each site. For Illinois and Kansas, our fungal mixtures included five species, and for Oklahoma our fungal mixtures included four species. Kansas cultures included Funneliformis mosseae, Funneliformis geosporum, Glomus mortonii, Rhizophagus diaphanous, and Claroideoglomus claroideum. Illinois cultures included F. mosseae, F. geosporum, G. mortonii, Septoglomus constrictum, and C. claroideum. Oklahoma cultures included S. constrictum, F. mosseae, Rhizophagus clarus, and Paraglomus occultum. Mesocosms were filled with 5 L of sterilized soil, inoculated with 150 mL of mixed AMF cultures from one study location spread evenly over the sterilized soil. Additional mesocosms were maintained as sterilized controls. Then mesocosms were capped with 2 L of sterilized soil. Fungi treatments were imposed in a fully factorial design with soil treatments (three soil types × four fungal treatments, including three fungi sources and a sterile control).
Plant treatments
We identified nine species of tallgrass prairie plants that occur near each of our study sites. These were chosen to represent a range of species life histories and known responsiveness to mycorrhizal fungi (Koziol and Bever 2015; Bauer et al. 2018). Plants and AMF can be locally adapted to each other, so we used seeds from Missouri, rather than from any of the locations that we isolated AMF from. We selected Missouri because it is an intermediate location between each of our study sites geographically and climatically. All seed was purchased from Hamilton Native Outpost (Missouri). Our study species included Elymus canadensis, Panicum capillare, Panicum virgatum, Sorghastrum nutans, Schizachyrium scoparium, and A. gerardii (Poaceae); Rudbeckia hirta and Liatris aspera (Asteraceae); and Monarda fistulosa (Lamiaceae). Seedlings of each were germinated in sterilized potting soil and allowed to grow for 2 weeks. Then a single individual of each species was transplanted into each mesocosm in a 3 × 3 grid. Arrangement of species along this grid was randomized for each of 10 study blocks.
Drought treatments
After transplanting, mesocosms were watered daily to field capacity for 4 weeks to allow seedlings to establish. Then, watering was reduced gradually over 2 weeks. For the remainder of the experiment, control mesocosms received 250 mL of water daily (3% of total soil volume), and the drought treatment received half of this (125 mL/day, 1.5% of total soil volume). We established additional mesocosms, maintained under similar conditions, to monitor soil moisture without disturbing experimental mesocosms. We confirmed that control treatments remained above 25% soil moisture, and the drought treatments remained between 15 and 20% soil moisture. These levels correspond to relatively high and low soil moisture conditions in tallgrass prairie, with well-documented effects on primary productivity (Knapp et al. 2001; Fay et al. 2003). Additionally, we monitored plants for signs of water stress. Plants in the drought treatment occasionally displayed outward signs of water stress (e.g. slight wilting, or curled leaves in the grasses), and minor adjustments were made to watering schedules to prevent soils from dropping below permanent wilting point during extremes in greenhouse conditions.
Data analysis
We tested for effects of soil, fungi, and drought treatments and their interactions on the total biomass of our mesocosms using an ANOVA, and we tested these effects on plant community composition using MANOVAs of the biomass of each species. In both cases, we used follow-up linear contrasts within the soil × fungi and soil × fungi × drought interactions terms to test for mycorrhizal mediation of plant adaptation to soil and drought conditions (following Thrall et al. 2002; Blanquart et al. 2013). That is local adaptation of AMF to their soil was measured and tested as the differential performance of plants in when AMF inocula matches their sympatric soil compared to their allopatric soil after accounting for the average growth responses to the inocula and the soils (i.e. the main effects). To compare results in mesocosms to expected results from tests on single seedlings, we compared mycorrhizal responsiveness and relative growth rates in this experiment to those reported in Bauer et al. (2018). This study compared growth of individual seedlings of our study species in soils inoculated with a diverse mix of AMF relative to controls in sterile soils. These regressions were limited to the six plant species for which data was available. Using linear regression, we also tested if mycorrhizal responsiveness in mesocosms predicted responsiveness to locally adapted fungi.
Results
Productivity
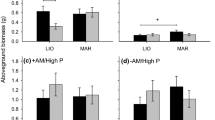
Soils from Oklahoma supported higher plant biomass, with intermediate biomass in soils from Illinois (p < 0.0001), and our drought treatment reduced aboveground biomass by 26% (p < 0.0001). Fungi treatments did not have significant main effects (Fig. 1) or interact with drought treatments (Fig. 2) to affect biomass. However, we did observe a soil × fungi interaction on plant biomass (p = 0.0013). Our a priori tests of local adaptation detected significant positive effects of local adaptation (p = 0.04), which were most pronounced in contrasts between soils from Oklahoma and our other two sites (p = 0.003, Fig. 1, Table 1, Appendix S1: Table 1). Drought did not significantly affect these measures of local adaptation.
Species composition
Our MANOVA results indicated that all main effects and interactions were significant in determining the species composition of our mesocosms (Table 2; Online Resource 1), though our follow up tests indicate responses varied substantially between plant species. Specific responses are detailed in Online Resource 1, and we focus here on the main effects of fungi, drought, and soil × fungi interaction and on tests of local adaptation within the soil × fungi and soil × fungi × drought interactions.
Seven of our nine study species had significant, positive responses to the presence of mycorrhizae, while E. canadensis (p < 0.0001) and P. capillare (p = 0.1) were negatively affected by mycorrhizal inoculation (Fig. 3a). Five of our study species responded negatively to our drought treatment, and four did not show a statistically significant response, although the aboveground biomass of all species except L. aspera was lower in the drought treatment (Fig. 3b). Despite significant effects of our drought and fungal treatments, we found only limited effects of AMF on plant responses to drought (linear contrasts “drought × fungi” Appendix Table 2). For most species, the effect of drought was not influenced by the presence or absence of AMF. For E. canadensis aboveground biomass was similar in drought and control treatments with fungi, but biomass was reduced by drought in sterile controls. However, E. canadensis in drought mesocosms without AMF was still over 20% larger than in mesocosms with AMF. For S. scoparium aboveground biomass was reduced by drought in inoculated mesocosm, but biomass was increased by drought in sterile mesocosm. In sterile control mesocosms biomass was consistently lower than in inoculated mesocosms for S. scoparium.
The effects of a AMF and b drought on the aboveground biomass (mean ± S.E.) of each of our study species. * Indicates significant treatment effects (p < 0.05). PC Panicum capillare, EC Elymus candensis, SN Sorghastrum nutans, PV Panicum virgatum, RH Rudbeckia hirta, MF Monarda fistulosa, AG Andropogon gerardii, SS Schizachyrium scoparium, LA Liatris aspera
We found evidence that local adaptation of mycorrhizae to soils lead to greater biomass for two of our study species: P. capillare and P. virgatum. In contrast, three species that benefitted from the presence of mycorrhizae overall (A. gerardii, S. scoparium, and S. nutans), experienced reduced benefits of this symbiosis when fungi were in “home” soils (Fig. 4). Unsurprisingly, E. canadensis generally responds negatively to the presence of AMF within a community context (Bauer et al. 2012, 2018), and in our current experiment we found that it did not respond to local adaptation of AMF. Our remaining species (R. hirta, L. aspera, and M.fistulosa) are generally responsive to the presence of AMF, but were not responsive to local adaptation of AMF (Appendix Table 2). Drought modified the effects of local adaptation between fungi and soil for three of our study species. For P. virgatum, measures of local adaptation were stronger in our drought treatment, and for A. gerardii and S. scoparium the negative effects of local adaption were reduced under drought conditions (Fig. 5).
Aboveground biomass (mean ± S.E.) of our study species in soils and fungi originating from Illinois (IL), Kansas (KS), and Oklahoma (OK) showing benefits of local adaptation to aPanicum capillare and bPanicum virgatum; negative effects of locally adapted AMF on dSorghastrum nutans, and eSchizachyrium scoparium, and fAndropogon gerardii; and non-significant effects on cElymus canadensis, gMonarda fistulosah, Rudbeckia hirta, and iLiatris aspera (full analyses in Appendix Table 2, linear contrast for “overall local adaptation”)
Aboveground biomass (mean ± S.E.) of a and bPanicum virgatum,c and dAndropogon gerardii, and e and fSchizachyrium scoparium with soil and fungi originating from Illinois (IL), Kansas (KS), and Oklahoma (OK) and in a, c, and e control and b, d, and f drought treatments showing effects of drought treatments on local adaptation
Species growth rates and mycorrhizal responsiveness within our mesocosm experiments was predicted by growth rates and responsiveness documented in tests of individual seedlings in past work. However, mycorrhizal responsiveness of our study species was not a significant predictor of those species’ response to local adaptation of AMF (Fig. 6).
Correlations between a AMF responsiveness and b growth rates in past measurements of individual seedlings in monoculture (Bauer et al. 2018) and on seedlings grown in mesocosms in this experiment. In a values were LN transformed to meet assumptions of the linear regression. In both cases responsiveness and growth rate in monoculture were good predictors of plant growth in mesocosms. However, c mycorrhizal responsiveness was not a good predictor of the benefits plants gain from locally adapted fungi
Discussion
We found evidence that local adaptation of mycorrhizal fungi (AMF) to soil conditions can affect the productivity of plant communities. Our study is unique in that we tested across multiple species of AMF from each site interacting with a diverse community of plant species, suggesting that local adaptation can be important within community contexts. This local adaptation effect was especially strong in contrasts of soils from Oklahoma to those originating from Kansas or Illinois, potentially because Oklahoma soils were distinct from the richer soils of Kansas and Illinois. The presence or absence of AMF did not affect community biomass, but our drought treatment did reduce biomass by 26%. This effect of drought had only weak interactions with our fungal treatments, indicating that local adaptation of AMF to soil characteristics is more important than adaptation to local climate for maintaining plant biomass.
The main effects of AMF inoculation on individual plant species were as expected from independent tests of plant species’ responsiveness to AMF (Bauer et al. 2018; Koziol and Bever 2015). Based on this past work, we expected that the plant species most responsive to mycorrhizae would also be the most responsive to local adaptation of AMF to soil conditions, since these species also tend to be most sensitive to the identity of the fungi they associate with (Koziol and Bever 2016). Contrary to this expectation, three grass species that are highly responsive to the AMF mutualism responded negatively to local adaptation of AMF, and two less-responsive grass species showed positive responses to local adaptation of AMF. Overall, we expected that local adaptation of AMF to soil conditions would favor the plant species most reliant on AMF, but we found the opposite pattern, with local adaptation favoring the plant species with the highest relative growth rate, despite its negative overall response to the presence of AMF as compared to sterilized controls.
We find this result especially surprising because of past work showing that local adaptation of AMF to soil conditions can have important benefits to plant species, including one of our study species, A. gerardii (Johnson et al. 2010). We suggest that our results differ from these past results due to the community context in which we tested these interactions, in contrast to past tests in monoculture conditions. Specifically, establishment of greenhouse mesocosms is most closely analogous to a highly disturbed environment with a very low density of established competitors. We should expect that these conditions would favor species with high relative growth rates. By rapidly achieving larger sizes, these fast-growing species would disproportionately influence the dynamics of mesocosms through asymmetric competition with slower growing competitors. Similarities can be drawn to a previous mesocosm study in which P. virgatum dominated the pots during the first year and this species sensitivity to inocula type drove the plant community diversity response to AM fungal composition and diversity (Vogelsang et al. 2006). In our experiment, P. capillare’s high relative growth rate and modest benefits derived from growing with locally adapted fungi appears to have been sufficient to reduce the benefits that more responsive plant species may have derived from associating with locally adapted fungi. We note that we were unable to separate below-ground biomass among species, and consequently, we have only reported on aboveground responses. We suggest that future research focus on local adaptation focus on belowground biomass and long-term responses of plant communities. It is quite possible that local AMF would most benefit the slower growing, more responsive plant species such as A. gerardii over a longer time frame than represented by this study.
We also note that local adaptation may occur between plants and their symbionts, and these effects may be more important than the adaptation of mycorrhizae to soil conditions (Rúa et al. 2016). Due to logistical constraints, we tested only for adaptation of AMF to their local soils and climate, and therefore restricted our plants to genotypes originating from Missouri, which was an intermediate location between all of our study sites. As measured by total biomass, we did see that plant communities received greater benefits from local adaptation of fungi to soil conditions, but we expect that these effects may be stronger if the plants are adapted to local fungi and to local soil conditions.
We expected that soil moisture would be an important component of local adaptation of AMF, with fungi originating from Kansas and Oklahoma delivering more benefits to plants under drought conditions. Our drought treatment did impose significant negative effects on our plants, with overall reductions in biomass and reduced growth in eight of our nine study species. However, AMF did not reduce the negative effects of drought. Recent meta-analyses suggest that AMF generally have an important role in plant water update, especially under drought conditions (Augé 2001; Delavaux et al. 2017) and prior studies of response of an individual AMF and plant species did show evidence of plants benefiting from drought adapted AMF in drought conditions (Stahl and Smith 1984), but our finding of weak AMF*drought interactions suggests that future work on the context-dependency of this aspect of the mycorrhizal symbiosis is warranted. Drought treatments interacted with measures of local adaptation, with the negative effects of local adaptation on S. scoparium and A. gerardii being reduced under drought conditions. Consistent with our arguments above, this may result from reductions in biomass of our fastest growing species, P. capillare, under drought conditions.
Our experimental conditions may be comparable to ecological restoration on highly disturbed soils, including restoration of land previously in row crops. Under these conditions, there is interest in incorporating soil microbial communities into ecological restoration to mitigate the effects of past land-use on the composition of soil microbial communities (Harris 2009; Bauer et al. 2017). This is of particular interest because the plant species that may benefit most from re-establishing microbial mutualisms also tend to be the late-successional species that are the target of ecological restoration (Koziol and Bever 2015; Bauer et al. 2018) and field trials have shown that late-successional species can benefit from locally-adapted relative to commercial AM fungi (Middleton et al. 2015). However, in our experiment, inoculation with locally adapted fungi favored dominance by a ruderal plant species at the expense of late-successional species that are more likely to be the focus of ecological restoration. We cannot anticipate how the outcomes of our short-term greenhouse experiment may translate to the long-term outcomes of ecological restoration. Nevertheless, our results highlight the context dependency of species interactions, and in practice, the re-establishment of microbial mutualisms may not always favor desirable plant species.
Our results suggest several avenues for future research on local adaptation of AMF. It was beyond the scope of this project to monitor AMF community responses to local adaptation, but it is likely that experimental conditions lead to changes in the total abundance and relative abundance of AMF species within our mesocosms. Investigating AMF responses to local adaptation could be especially interesting if tests including locally adapted plant genotypes were also included. We also suggest that the mechanisms underlying local adaptation warrant further investigation. It is possible that each fungi population is individually adapted to local conditions, so that any individual fungal isolate would generate patterns similar to those we observed. It is also possible that complementary among co-occurring fungal taxa may have evolved so that local adaptation is an emergent property of the community.
Overall, we found support for the hypothesis that adaptation of AMF to local soil conditions increase the benefits these fungi deliver to plants. This effect was evident in measures of total plant community biomass. Unexpectedly, the presence of AMF did not appear to mitigate the negative effects of drought. We did see that local adaptation of AMF to soil conditions can lead to shifts in plant community composition. However, individual species showed surprising responses to local adaptation of AMF, with two plant species that are relatively less responsive to AMF showing benefits of associating with locally adapted fungi and plant species that are more responsive to AMF being negative affected by local adaptation of their fungal symbionts. This pattern likely emerged because high relative growth rates of non-responsive plant species allowed these ruderal species to dominate our experimental mesocosms, overwhelming the potential positive effects of local adaptation of fungi on the plant species that are most reliant on this mutualism.
References
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. https://doi.org/10.1007/s005720100097
Barrett LG, Broadhurst LM, Thrall PH (2012) Geographic adaptation in plant-soil mutualisms: tests using Acacia spp. and rhizobial bacteria. Funct Ecol 26:457–468. https://doi.org/10.1111/j.1365-2435.2011.01940.x
Bauer JT, Kleczewski NM, Bever JD, Clay K, Reynolds HL (2012) Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi, and the productivity and structure of prairie grassland communities. Oecologia 170:1089–1098. https://doi.org/10.1007/s00442-012-2363-3
Bauer JT, Blumenthal N, Miller AJ, Ferguson JK, Reynolds HL (2017) Effects of between-site variation in soil microbial communities and plant-soil feedbacks on the productivity and composition of plant communities. J Appl Ecol 54:1028–1039. https://doi.org/10.1111/1365-2664.12937
Bauer JT, Koziol L, Bever JD (2018) Ecology of floristic quality assessment: testing for correlations between coefficients of conservatism, species traits and mycorrhizal responsiveness. AoB Plants. https://doi.org/10.1093/aobpla/plx073
Blanquart F, Kaltz O, Nuismer SL, Gandon S (2013) A practical guide to measuring local adaptation. Ecol Lett 16:1195–1205. https://doi.org/10.1111/ele.12150
Delavaux CS, Smith-Ramesh LM, Kuebbing SE (2017) Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 98:2111–2119. https://doi.org/10.1002/ecy.1892
Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2003) Productivity responses to altered rainfall patterns in a C 4-dominated grassland. Oecologia 137:245–251. https://doi.org/10.1007/s00442-003-1331-3
Harris J (2009) Soil microbial communities and restoration ecology: facilitators or followers? Science 325:573–574. https://doi.org/10.1126/science.1172975
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195
Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci 107:2093–2098. https://doi.org/10.1073/pnas.0906710107
Knapp AK, Briggs JM, Koelliker JK (2001) Frequency and extent of water limitation to primary production in a mesic temperate grassland. Ecosystems 4:19–28. https://doi.org/10.1007/s100210000057
Koziol L, Bever JD (2015) Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology 96:1768–1774. https://doi.org/10.1890/14-2208.1
Koziol L, Bever JD (2016) AMF, phylogeny, and succession: specificity of response to mycorrhizal fungi increases for late-successional plants. Ecosphere 7:e01555. https://doi.org/10.1002/ecs2.1555
Koziol L, Bever JD (2017) The missing link in grassland restoration: arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J Appl Ecol 54:1301–1309. https://doi.org/10.1111/1365-2664.12843
Lau JA, Lennon JT (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci 109:14058–14062. https://doi.org/10.1073/pnas.1202319109
Middleton EL, Bever JD (2012) Inoculation with a native soil community advances succession in a grassland restoration. Restor Ecol 20:218–226. https://doi.org/10.1111/j.1526-100X.2010.00752.x
Middleton EL, Richardson S, Koziol L, Palmer CE, Yermakov Z, Henning JA, Schultz PA, Bever JD (2015) Locally-adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere 6:276. https://doi.org/10.1890/ES15-00152.1
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291. https://doi.org/10.1890/02-0298
Rúa MA, Antoninka A, Antunes PM, Chaudhary VB, Gehring C, Lamit LJ, Piculell BJ, Bever JD, Zabinski C, Meadow JF, Lajeunesse MJ, Milligan BG, Karst J, Hoeksema JD (2016) Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol Biol 16:122. https://doi.org/10.1186/s12862-016-0698-9
Sanford WE, Selnick DL (2013) Estimation of evapotranspiration across the conterminous united states using a regression with climate and land-cover data1. J Am Water Resour Assoc 49:217–230. https://doi.org/10.1111/jawr.12010
Schultz PA, Miller RM, Jastrow JD, Rivetta CV, Bever JD (2001) Evidence of a mycorrhizal mechanism for the adaptation of Andropogon gerardii (Poaceae) to high- and low-nutrient prairies. Am J Bot 88:1650. https://doi.org/10.2307/3558410
Sherrard ME, Maherali H (2012) Local adaptation across a fertility gradient is influenced by soil biota in the invasive grass, Bromus inermis. Evol Ecol 26:529–544. https://doi.org/10.1007/s10682-011-9518-2
Stahl PD, Smith WK (1984) Effects of different geographic isolates of Glomus on the water relations of Agropyron smithii. Mycologia 76:261–267. https://doi.org/10.1080/00275514.1984.12023835
Thrall PH, Burdon JJ, Bever JD (2002) Local adaptation in the Linum marginale Melampsora lini host-pathogen interaction. Evolution 56:1340–1351. https://doi.org/10.1111/j.0014-3820.2002.tb01448.x
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of the tallgrass prairie system. New Phytol 172:554–562. https://doi.org/10.1111/j.1469-8137.2006.01854.x
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738. https://doi.org/10.2307/2446507
Acknowledgements
This work was supported by Strategic Environmental Research and Development Program grant RC-2330 and National Science Foundation grants 1556664 and 1656006 to JDB and United States Department of Agriculture–National Institute of Food and Agriculture Grant 2016-67012-24680 to JTB. We appreciate assistance with greenhouse work from Ying “Bank” Xuen Hoe, Vanessa Snyder, and Alex Varney.
Author information
Authors and Affiliations
Contributions
JTB, LK, and JDB designed the experiment. JTB and LK established the experiment. JTB collected and analyzed data. JTB wrote the manuscript, with feedback and revisions from LK and JDB.
Corresponding author
Ethics declarations
Conflict of interest
Liz Koziol is the owner of Mycobloom LLC.
Additional information
Communicated by Sarah M. Emery.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bauer, J.T., Koziol, L. & Bever, J.D. Local adaptation of mycorrhizae communities changes plant community composition and increases aboveground productivity. Oecologia 192, 735–744 (2020). https://doi.org/10.1007/s00442-020-04598-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04598-9