Abstract
Invasive plants may outcompete and replace native plant species through a variety of mechanisms. Recent evidence indicates that soil microbial pathways such as pathogen accumulation may have a considerable role in facilitating competition between native and invasive plants. To assess microbe-mediated pathways of invasion, we tested the impacts of invaded and non-invaded field soils on plant establishment using naturally occurring populations of the common Eurasian invader Cirsium arvense (Canada thistle) in Southern Ontario, Canada. Linked field and greenhouse experiments were used to quantify differences in the germinability and early growth rates of native plant species, depending on exposure to the microbial community in invaded or non-invaded soils. The invaded microbial community significantly reduced early growth rates for two of the seven native species surveyed, and decreased seed germination for another. In contrast, the germination and growth of invasive Cirsium were not affected by its own soil microbial community. These results demonstrate that the invasion of C. arvense can reduce the performance of some native plant species through changes to the soil microbial community. Different effects on different species suggest that this invader may also change the relative importance of certain natives in the invaded community. If these effects influence plant abundance in the field, microbially mediated interactions in the soil may aid the invasion of C. arvense and facilitate the disruption of invaded communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All plant species cultivate a unique community of soil organisms (Bever et al. 1997; Bezemer et al. 2006), and, in turn, soil communities influence plants through complex networks of association that range along a continuum from mutualist to pathogenic (Johnson et al. 1997; Dickie et al. 2017). These feedbacks between plants and soil biota have an important influence on plant success (Bever 1994; Bever et al. 1997; Kulmatiski et al. 2008; van der Putten et al. 2013; Kulmatiski et al. 2016) and can moderate interspecific competition (van der Putten and Peters 1997; Bever 2003) as well as alter plant community composition, diversity, and succession (Klironomos 2002; van der Heijden et al. 2008; Bever et al. 2012; Li et al. 2014). Such feedbacks can also influence the success of invasive plant species: recent research indicates that interactions with soil microbes can both facilitate and inhibit plant invasions (Inderjit and van der Putten 2010; Dunn et al. 2012; van der Putten et al. 2013; Bardgett and van der Putten 2014; Dickie et al. 2017).
As well as directly affecting the invader itself, changes in soil biota caused by the introduction of a species may alter interactions between invasive and native plant species (van der Putten et al. 2007; Rodríguez-Echeverría et al. 2009; Inderjit and van der Putten 2010; Gibbons et al. 2017). Such changes in soil microbial communities in response to invaders may represent a critical, but still poorly known, impact of invasive species (Wolfe and Klironomos 2005). For instance, invasive plants may inhibit competitors through the accumulation and spread of pathogens to which native plants are susceptible (Eppinga et al. 2006). The influence of pathogen accumulation on competition in plant communities is well-documented (e.g., van der Putten and Peters 1997; Packer and Clay 2003; Reinhart and Callaway 2006), and an increasing number of studies have provided evidence for the importance of such effects in invasive plant systems (e.g., van Grunsven et al. 2007; Mangla et al. 2008; de la Peña et al. 2010; Meng et al. 2014—but see Del Fabbro and Prati 2015). The impacts of shared pathogens are also dependent on host susceptibility: if exotic plants are more susceptible to a given pathogen, accumulation may instead limit their abundance via biotic resistance (Nijjer et al. 2007; Diez et al. 2010). Invaders may also affect the soil communities upon which natives depend by disrupting native mutualisms (Stinson et al. 2006; Hale et al. 2011; Dickie et al. 2014; Grove et al. 2017) or by promoting their own (Callaway et al. 2004; Richardson et al. 2000; Moeller et al. 2015). Given the complex role of soil ecology in plant invasions (Dickie et al. 2017) and the limited number of systems in which ecologists have tested this (Li et al. 2014; Preston et al. 2016), the influence of soil microbial processes in many major plant invasions is unknown.
In this study, we test whether an abundant invader, Canada thistle (Cirsium arvense (L.) Scop.—Asteraceae), may facilitate its own invasion by promoting a soil community detrimental to the regeneration of co-occurring natives. We focused on establishment characters, because in many cases, the population and community impacts of soil microbes are strongest during the critical early development stages of plants (Templeton and Levin 1979; Blaney and Kotanen 2001; Packer and Clay 2003; Rasmussen et al. 2015; Crocker et al. 2016). We combine field and greenhouse experiments to investigate whether soils conditioned by Cirsium arvense reduce germination of competitors’ seeds or inhibit the growth of seedlings following germination.
Materials and methods
Study site and species
Field components of our experiments were conducted in old fields at the University of Toronto’s Koffler Scientific Reserve (KSR; 44°02′N 79°32′W), in King Township, ON, Canada. Greenhouse components were conducted at the University of Toronto, Mississauga, ON, Canada.
Our focal invader, the perennial herb Cirsium arvense (hereafter referred to as Cirsium) is a Eurasian native now present in most temperate regions globally (Moore 1975; Cripps et al. 2011; Guggisberg et al. 2012), and is considered one of the most damaging invasive plant species worldwide (Tiley 2010). Cirsium has been present in North America for hundreds of years (Moore 1975; Holm et al. 1977) and is common at our study site. The effects of Cirsium on soil biota have received minimal attention, besides dated evidence indicating potential allelopathic effects (Bendall 1975; Wilson 1981); however, Nunes and Kotanen (2018) have recently demonstrated that Cirsium is susceptible to negative soil feedback in the field.
The other species selected for study (Table 1) are common Ontario natives that frequently occur in habitats invaded by Cirsium, including old fields at our study site (though Lactuca biennis is more typical of shaded sites, and Eutrochium maculatum occurs in wet areas). Due to the increased likelihood of shared symbionts between closely related species (Parker et al. 2015), these natives were selected to span a range from phylogenetically distant to closely related Asteraceae confamilials of Cirsium. In summer and fall 2016, seeds were collected from multiple wild populations of these species across Southern Ontario, Canada. For each species, seeds from different populations were air-dried and mixed into one combined seed pool.
Soil sampling
In October 2016, field soil was sampled from eight sites (20 m × 20 m) at KSR. Sites were separated by at least 90 m as well as physical barriers such as streams, forests, wetlands, or roads. Within each site, soil was sampled to a depth of 15 cm from four points within 1 m2 plots, both inside (> 40 stems m−2) and outside (zero stems within 2 m radius) Cirsium populations. These soil samples were, respectively, classified as either “invaded” or “non-invaded”. To reduce contamination by wild seeds, the top centimetre of each sample was discarded. Samples were stored separately in ambient, outdoor conditions until use. Prior to immediate use, soil samples were sieved to remove rocks and large plant material.
Experiment 1: seed germination
A seed burial field experiment was used to investigate how Cirsium may impact the emergence of competitors from the seed bank. The survival of seeds in invaded and non-invaded soils was assessed across eight sites, with a factorial soil fungicide treatment to test whether any effects could be explained by soil fungi. In addition to Cirsium, seven locally abundant native species were used: Asclepias syriaca, Lactuca biennis, Monarda fistulosa, Poa pratensis (native in part), Solidago canadensis, and Symphyotrichum puniceum (Table 1).
In November 2016, recently collected seeds were mixed with 20 mL of sieved field soil in bags constructed from nylon stockings. Each bag contained 20 seeds from a single plant species (visually assessed for viability and not frozen before use) and invaded or non-invaded soil (the “invasion” treatment) from one of the eight sites. Seed bags were then saturated in either an aqueous fungicide solution or a water control in a factorial design, resulting in four types of soil in seed bags: invaded live, invaded fungicide, non-invaded live, and non-invaded fungicide. The fungicide used was Captan (2.4 g L−1 50% WP; TreeHelp, Toronto, ON; concentration as recommended by the manufacturer for seed and soil treatments). Captan is a broad-spectrum fungicide that has been successfully used in similar seed-protection experiments (O’Hanlon-Manners and Kotanen 2004; MacKay and Kotanen 2008); in a separate trial, this fungicide was not found to have any direct, unintended effect on seed germination rates for any of the plant species used in this experiment (Supplement S-1). Although fungicide treatment is unlikely to kill all soil fungi (or other microbes), this represents a relatively long-lasting treatment for pathogen reduction, which is appropriate given the continuous exposure of seed bags to soil microbiota throughout this experiment.
These seed bags were then buried in the field to overwinter for 6 months under natural conditions. This timing was established to mimic the natural duration of many seeds’ post-dispersal exposure to soil fungi. Each bag was buried two centimetres below the soil surface at the source of the original soil sample. At each site (n = 8), two replicate bags (n = 2) of each fungicide treatment (n = 2) were buried in both invaded and non-invaded areas. This design resulted in a total of 64 bags per species (n = 7), each containing 20 seeds.
At the end of the 6-month burial period, seed bags were recovered from the field, excepting two containing Asclepias syriaca which could not be located. The contents of bags were spread over sterilized potting mix (Sunshine Mix #1, autoclaved for two 50-min cycles at 121 °C) in 10 cm × 13 cm cells. Seedlings that had already germinated in their bags were removed and counted. Over an 11-week period, seeds were kept moist in a greenhouse (60% relative humidity, 14 h daily lighting, and 17/10 °C day/night temperature cycle). Emerging seedlings were removed and counted daily. The total number of germinated seeds was summed for each sample.
Experiment 2: seedling growth
We used soil sampled from field populations of Cirsium to estimate the feedback effect caused by natural conditioning of the soil (Bever et al. 1997; Kulmatiski et al. 2008; Pernilla Brinkman et al. 2010). In this greenhouse study, the growth of seedlings grown in soil conditioned by invasive Cirsium is compared with that of plants grown in non-invaded field soil. Soil sterilization was used to distinguish between biotic and abiotic effects of the conditioned soil community.
Seedling growth rates were measured for seven native species, plus Cirsium. Species were chosen based on germinability under greenhouse conditions, as well as the criteria listed above. In addition to Cirsium, plant species used were: Erigeron canadensis, Eutrochium maculatum, Lactuca biennis, Monarda fistulosa, Poa pratensis, Solidago canadensis, and Symphyotrichum puniceum (Table 1). Seeds from each of these species were surface-sterilized using 95% ethanol, cold stratified (4 °C) on moist filter paper for 5 weeks, and planted on sterilized potting soil. They were kept in spring-like greenhouse conditions (60% relative humidity, 14 h daily lighting, and 17/10 °C day/night temperature cycle) which were maintained for the duration of the experiment.
Upon germination, seedlings at the cotyledon stage were individually transplanted into conical tubes (SC10L Super cells, 250 mL, Stuewe & Sons). To account for initial size differences, cotyledon leaf length was measured (mm) prior to transplanting. Any seedlings that died within 3 days of transplanting were replaced. Each container was filled with 230 mL of sterilized potting soil and 10 mL of sieved field soil inoculum from a single site. This proportion of inoculum represents about 4% of container volume; larger volumes are more likely to introduce unwanted changes in soil composition (Vandegehuchte et al. 2010). Each inoculum was (1) derived from soil either invaded or not-invaded by Cirsium (the “invasion” treatment) and (2) either live or sterilized by autoclaving (the “sterilization” treatment). Crossing invasion × sterilization treatments resulted in four soil treatments: invaded live, invaded sterile, non-invaded live, and non-invaded sterile. These tests for both the soil-mediated effects of invasion, and, by sterilizing half of each field soil treatment, for whether the observed impacts are biotic or abiotic. Since beneficial mycorrhizae can be suppressed in relatively rich media such as potting mix (Schroeder and Janos 2004; Treseder 2004; Balzergue et al. 2013), these methods may underestimate their importance, but should be correspondingly sensitive to detecting effects of pathogens.
For each plant species tested, each combination of invasion × sterilization treatments was replicated by field soil source (n = 6) and by blocks within the greenhouse (n = 10). Individuals were randomized within blocks at the time of transplanting, and blocks were re-arranged weekly within the greenhouse for the duration of the 45-day experiment. This design resulted in a total of 60 replicate seedlings per soil treatment (n = 4), per species (n = 8), for a total of 1920 experimental units, each containing a single seedling.
Throughout the experiment, seedling height (mm) was measured every 10 days. At the end of the 45-day growth period, above- and belowground plant biomass were separately harvested, dried at 60 °C for 48 h, and weighed (mg). Above- and belowground biomass measures were made twice for each individual seedling. Any individuals whose repeated biomass measurements varied by > 2 mg were re-weighed. All measurements were made blind to soil treatment.
Statistical analysis
Analyses were performed in R (version 3.4.1; R Core Team 2017). In both experiments, plant performance measures were independently analyzed for each plant species.
Germination data
Prior to analysis, we assessed the normality and homoscedasticity of germination rate data and their residuals. Since germination data departed marginally from normality, log and square-root transformations were tested, but ultimately, the non-transformed data were closest to meeting model assumptions. Results should, therefore, be interpreted with some caution, although statistical analyses using alternative transformations and links (e.g., poisson, binomial, and lognormal) produced similar results.
To test variation in germination rates between soil treatments, we applied generalized linear mixed models (GLMMs) for each species using restricted maximum likelihood (lme4 package, version 3.4.1; R Core Team 2017). Models contained four independent variables, with soil invasion status and fungicide treatment as fixed effects, and sample nested within site as hierarchical random effects. GLMMs were fit to germination data using the lmer function with a normal distribution and identity link. This model estimates the size and direction of effect. Maximum likelihood ratio (LR) tests were then used to determine the significance of main effects and interactions.
Growth data
Due to a close linear relationship between above- and belowground biomass, we used total biomass as our primary measure of size. To meet assumptions of homoscedasticity and normality of residuals, total biomass data were square-root transformed. Results for S. canadensis growth should be interpreted with some caution, as square-root-adjusted biomass still did not meet homogeneity of variance assumptions in this species.
Between-treatment differences in total biomass were assessed for each plant species using GLMMs with restricted maximum likelihood (lme4 package). Similar to the germination experiment, soil invasion status and sterilization treatments were included in the model as fixed effects. Random effects were greenhouse blocks, soil sampling site, and initial size at transplanting. Data were again fit to the model using the lmer function (lme4 package) with a normal distribution and identity link, and ANOVA-based maximum LR tests were used to determine the significance of main effects and interactions.
Results
Seed germination
Both soil invasion status and fungicide treatment influenced germination; however, there were differences in responses among species (Fig. 1). Asclepias syriaca emergence was higher in fungicide-treated soil (\(\chi_{1}^{2}\) = 4.35, p < 0.05; fixed effect = 2.14 seeds), but not significantly affected by soil invasion status (\(\chi_{1}^{2}\) = 0.31, p = 0.58) or the interaction of fungicide and invasion treatments (\(\chi_{1}^{2}\) = 1.75, p = 0.19). In contrast, germination rates for Solidago canadensis were significantly higher in live soil (\(\chi_{1}^{2}\) = 4.43, p < 0.05, fixed effect = 2.38 seeds), and in invaded soil (\(\chi_{1}^{2}\) = 7.71, p < 0.01, fixed effect = 3.19 seeds). The interaction of fungicide and invasion treatments did not influence germination in this species (\(\chi_{1}^{2}\) = 0, p = 1). Soil invasion (\(\chi_{1}^{2}\) = 0.12, p = 0.73) and fungicide treatment (\(\chi_{1}^{2}\) = 2.03, p = 0.15) did not have significant main effects on germination of Lactuca biennis; however, these treatments did significantly interact (\(\chi_{1}^{2}\) = 5.21, p < 0.05; Fig. 1). Invasion status did not influence this species’ germination in live (\(\chi_{1}^{2}\) = 2.98, p = 0.08) or fungicide treatments (\(\chi_{1}^{2}\) = 1.73, p = 0.19). Similarly, in non-invaded soils, fungicide treatment did not influence germination (\(\chi_{1}^{2}\) = 0.58, p = 0.45). However, in invaded soils, L. biennis seedling emergence was significantly higher in samples treated with fungicide (\(\chi_{1}^{2}\) = 7.81, p < 0.01; fixed effect = + 4.63 seeds; Fig. 1). For Cirsium arvense, Monarda fistulosa, Poa pratensis, and Symphyotrichum puniceum, invasion and fungicide soil treatments had no effect on germination rate (all p > 0.09; Fig. 1).
Effects of soil invaded by Cirsium arvense on seed germination of aAsclepias syriaca, bCirsium arvense, cLactuca biennis, dMonarda fistulosa, ePoa pratensis, fSolidago canadensis, and gSymphyotrichum puniceum. Germination was measured as the number of seeds that emerged out of 20 seeds per sample. Bars display means (± SE; n = 16 per treatment combination) for the crossed effects of soil invasion status and fungicide treatment. Significant effects and their interactions, as indicated by generalized linear mixed models, are listed for each species
Seedling growth
Biomass data were square root transformed to meet assumptions of residual normality and homogeneity of variance in the analysis; however, for the reporting of results, these data have been back-transformed for clarity and interpretability.
Total plant biomass was significantly higher in sterilized soil for all species tested (p < 0.001; Fig. 2). However, the effects of random factors (soil sampling site, size at transplanting, and greenhouse block) differed among species (Supplement).
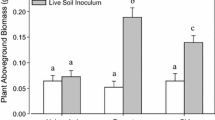
Biotic effects of soil invaded by Cirsium arvense on seedling growth, measured as total dry biomass in plant species aCirsium arvense, bErigeron canadensis, cEutrochium maculatum, dLactuca biennis, eMonarda fistulosa, fPoa pratensis, gSolidago canadensis, and hSymphyotrichum puniceum. Bars display means (± SE; n = 60 per treatment combination) for the crossed effects of soil invasion status and sterilization treatment. Significant effects and their interactions, as indicated by generalized linear mixed models, are listed for each species, except for the positive effect of soil sterilization which was significant (p < 0.001) for all test species
In four species, soil invasion positively affected biomass (Fig. 2). Relative to plants grown in non-invaded soil, the biomass of Cirsium plants grown in invaded soil was 20.4% higher (\(\chi_{1}^{2}\) = 7.03, p < 0.01) on average. Similar increases were observed in invaded soil treatments for species E. canadensis, E. maculatum, and L. biennis, where biomass increased by 17.5% (\(\chi_{1}^{2}\) = 6.06, p < 0.05), 29.2% (\(\chi_{1}^{2}\) = 5.79, p < 0.05), and 20.8%, respectively (\(\chi_{1}^{2}\) = 8.31, p < 0.01). This increase was not dependent on sterilization, as shown by the non-significant interaction of sterilization and invasion status for these species (Cirsium: \(\chi_{1}^{2}\) = 1.52, p = 0.22; Erigeron: \(\chi_{1}^{2}\) = 0.56, p = 0.46; Eutrochium: \(\chi_{1}^{2}\) = 0.02, p = 0.89; Lactuca: \(\chi_{1}^{2}\) = 0.24, p = 0.62).
In contrast, for M. fistulosa and S. canadensis, soil invasion status had no general influence on biomass (Monarda:\(\chi_{1}^{2}\) = 0.28, p = 0.60; Solidago:\(\chi_{1}^{2}\) = 0.33, p = 0.57), but did significantly interact with sterilization treatments (Monarda:\(\chi_{1}^{2}\) = 10.29, p < 0.01; Solidago:\(\chi_{1}^{2}\) = 5.13, p < 0.05). The effects of sterilization were, therefore, assessed independently in both invaded and non-invaded soils using GLMMs and LR tests. Given the strong sterilization-associated increase in biomass across all species and treatments (Fig. 2), it is unsurprising that sterilization significantly affected biomass in both invaded (Monarda:\(\chi_{1}^{2}\) = 82.41, p < 0.001; Solidago:\(\chi_{1}^{2}\) = 114.90, p < 0.001) and non-invaded soils (Monarda:\(\chi_{1}^{2}\) = 22.25, p < 0.001; Solidago:\(\chi_{1}^{2}\) = 60.52, p < 0.001). However, the magnitude of this positive sterilization effect appears to be larger in invaded soils (fixed effect: Monarda = + 170.5%; Solidago = + 255.0%) compared to that in non-invaded soils (fixed effect: Monarda = + 59.8%; Solidago = + 131.2%; Fig. 2).
The effects of invasion status were assessed independently in both sterilized and live soils for these species. When the inoculum was sterile, plants grown in invaded soils had significantly (Monarda:\(\chi_{1}^{2}\) = 7.53, p < 0.01; Solidago:\(\chi_{1}^{2}\) = 4.65, p < 0.05) higher biomass than those grown in non-invaded soils (fixed effect: Monarda = + 20.0%; Solidago = + 11.4%). However, when inoculum was live, there was a non-significant difference between biomass in invaded and non-invaded soils (Monarda:\(\chi_{1}^{2}\) = 3.74, p = 0.053; Solidago:\(\chi_{1}^{2}\) = 3.12, p = 0.077). This may suggest contrasting impacts of invasion in live and sterilized soil (Fig. 2). Notably, if these marginally non-significant effects were significant, the impact of invasion on biomass would be negative in live soils (fixed effect: Monarda = − 35.0%; Solidago = − 27.5%), which remains in contrast to the effect of invasion in sterilized soil.
Finally, soil invasion status did not significantly alter biomass in P. pratensis (\(\chi_{1}^{2}\) = 2.57, p = 0.11) or S. puniceum (\(\chi_{1}^{2}\) = 0.85, p = 0.36), nor did the interaction between invasion and sterilization (Poa: \(\chi_{1}^{2}\) = 2.39, p = 0.12; Symphyotrichum:\(\chi_{1}^{2}\) = 1.72, p = 0.19).
Discussion
Recent evidence indicates that interactions between invasive plants and soil biota have the potential to facilitate competition, alter native plant communities, and influence invasion (Dunn et al. 2012; Bardgett and van der Putten 2014; Dickie et al. 2017). Here, we demonstrate that the soil microbial community associated with invasive Cirsium arvense negatively influences the germination and seedling growth of some native plant species, but not the invader itself. We also found evidence that the physical or chemical changes in Cirsium-invaded soils may positively impact certain species. Together, these differential effects on co-existing plant species have the potential to alter the composition of invaded communities, and possibly to increase abundance of thistle relative to more vulnerable competitors.
Effects of Cirsium on seed germination
Germination of L. biennis seeds was significantly reduced by the fungal soil community, as indicated by the improved germination of fungicide-treated seeds. This effect only occurred in invaded soils, implicating Cirsium-associated fungi as the cause. In contrast, germination of the other five native species tested (A. syriaca, M. fistulosa, P. pratensis, S. canadensis, and S. puniceum), and of Cirsium itself, apparently was not altered by Cirsium-associated fungi. The reduction in L. biennis seed germination may be due to the accumulation of fungal pathogens in habitats invaded by Cirsium: the role of fungal pathogens in seed death is well-documented (Schafer and Kotanen 2004; Kotanen 2007; Mangla et al. 2008), and invader-induced changes to seed pathogen populations have also been shown (Orrock et al. 2012). Any such Cirsium-associated pathogens evidently did not affect germination of the other species tested; however, co-occurring plant species can differ strongly in their susceptibility to seed pathogens (Schafer and Kotanen 2004). L. biennis may be especially poorly defended, or may be particularly vulnerable to attack by a host- or habitat- specific fungus. L. biennis is less likely than most of our experimental species to occur in old fields; possibly, it is less adapted to pathogens occurring there. In contrast with the habitat-dependence exhibited by L. biennis, germination of A. syriaca was improved by fungicide treatment in all habitats, not just sites dominated by Cirsium. This may indicate susceptibility to widespread pathogens not associated with Cirsium. Finally, germination of S. canadensis seeds was significantly higher in invaded soils. This result was not influenced by fungicide addition, suggesting that it may be due to abiotic effects such as greater soil nutrient availability in invaded areas (discussed further below), or possibly the effects of non-fungal soil biota (Nunes and Kotanen 2018). Regardless of mechanism, the selective suppression or promotion of different plant species has the potential to alter the composition of invaded communities.
In this experiment, the measured impacts of soil fungi may have been conservative for a number of reasons. First, although Captan is a broad-spectrum fungicide (Torgeson 1969), it is unlikely to be equally effective against all fungi. Second, seed bags were buried for a period of 6 months. Over this period, the concentration of fungicide likely declined as it leached from the seed bags or was degraded, though Captan can maintain efficacy even at low concentrations (Gupta 2010). Third, the Captan concentration used was lower than in similar experiments (e.g., O’Hanlon-Manners and Kotanen 2004; MacKay and Kotanen 2008), though it was the concentration recommended by the manufacturer for seed and soil treatments. For all of these reasons, the treatment used is unlikely to have completely excluded fungal pathogens, reducing the magnitude of observed effects. Finally, some of the seeds that failed to emerge may have been dormant rather than dead. We analyzed species separately to control for any interspecific variation in seed dormancy rates; nonetheless, any dormant seeds counted as non-emergent would make intraspecific treatment effects less pronounced.
Effects of Cirsium on seedling growth
Seedlings of all species tested grew significantly larger in sterilized soil, including those of Cirsium itself; there was no suggestion that the one exotic in our data set (Cirsium) was less susceptible to pathogens than its native competitors, in contrast with some other studies (e.g., van Grunsven et al. 2007; Engelkes et al. 2008). The reduced growth in live soil treatments, observed for inoculum from both invaded and non-invaded sites, may be due to an overall negative effect of the soil microbial community. Negative effects such as these are common under natural conditions (van der Putten et al. 1993; Bever et al. 1997; Klironomos 2002). It is worth noting that soils with high nutrient concentrations can reduce plants’ mycorrhizal dependence (Schroeder and Janos 2004; Treseder 2004; Balzergue et al. 2013). Since nutrient-rich potting mix (Supplement) comprised 96% of growth soil volume in this experiment, the observed feedbacks may understate the positive impacts of mycorrhizal associations in live field soils, instead enhancing the detection of pathogens. However, Nunes and Kotanen (2018) have previously demonstrated negative effects of field soil biota on Cirsium arvense at this site. In principle, the observed effect of soil sterilization could also reflect unintended by-products of the autoclaving process, such as altered soil structure or increased nutrient availability (Berns et al. 2008); we added only a small amount (4%) of sterile or non-sterile inoculum to each pot to guard against this possibility.
Invasion by Cirsium had unexpected consequences for several species. Seedlings of four species (E. canadensis, E. maculatum, L. biennis, as well as Cirsium) grew slightly larger in treatments inoculated with Cirsium-invaded soil regardless of sterilization; this effect was small compared to the effect of sterilization, but still significant. Interestingly, all four of these species are in the same family (Asteraceae), perhaps suggesting some phylogenetically conserved trait contributed to this response. This result is inconsistent with dated evidence that Cirsium can negatively affect neighbours by allelopathy (Bendall 1975; Wilson 1981; Tiley 2010). Instead, this suggests that invasion positively affects growth through an abiotic pathway. In principle, one possibility might be changes in the nutrient availability of Cirsium-invaded soils. Invasive species can alter soil nutrients by organic inputs such as leaf litter (Allison and Vitousek 2004), or by altering the community of microbial decomposers (Wolfe and Klironomos 2005); in either case, the abiotic legacy of these soil changes might persist despite soil sterilization. Alternatively, these results might indicate thistles preferentially grow in richer sites that provide more nutrient-rich inocula: there is evidence that Cirsium prefers nutrient-rich soils (Fairbairn and Thomas 1959; but see Locket 1946; Moore 1975). Nunes and Kotanen (2018) did find evidence in the field that Cirsium grows slightly better in proximity to conspecifics despite increased herbivore pressure, perhaps indicating better soil quality in invaded sites. However, both these explanations seem unlikely in view of the small amount of inoculum used, and the close proximity and similarity of the invaded and uninvaded sites sampled. Two plant species also showed evidence of a biotic effect of Cirsium invasion. Growth of M. fistulosa and S. canadensis seedlings depended on an interaction between sterilization and soil source: sterilization improved growth more in soils sampled from invaded sites than it did in soils from uninvaded sites. This suggests that Cirsium may accumulate pathogens harmful to these competitors, as has been proposed for other invaders (Eppinga et al. 2006; Mangla et al. 2008).
Implications of invasion-altered soil effects
The regeneration phase is critical for the population biology of many plants, for which seed and seedling death often comprise the bulk of mortality (Harper 1977; Crawley 2000). Together, our experiments found evidence of negative effects of soil biota on the germination of some species, and on the seedling growth of all. We also found evidence that these biotic effects were enhanced by the presence of soil conditioned by Cirsium for the germination of one species (L. biennis) and for the growth of two others (M. fistulosa and S. canadensis). Other species showed other responses, but in no case were the effects of soil biota improved by the presence of soil conditioned by Cirsium. For species that were not negatively affected by Cirsium-associated soil biota, we often found evidence of an unexplained abiotic benefit of invaded soils on growth. Even this effect may ultimately be the result of physical or chemical changes to soil caused by Cirsium-associated decomposers or mutualists.
Given the specificity of many plant–microbe associations (Klironomos 2003; Aleklett et al. 2015; Lebeis 2015), it is unsurprising that invasion-induced changes in soil biology affect plant species differently. Although we found evidence of an effect of Cirsium on only some of its potential competitors, this still implies that soil changes induced by this invader may impact the composition of communities it invades by differentially affecting different species. Many other species in these diverse old-field communities may be impacted in ways that are similar to the effects observed in this study. In addition, one of the species that did experience a negative biotic effect, Solidago canadensis, is an extremely important matrix-forming plant in these communities. Suppression of this species by Cirsium-associated microbes could significantly alter the character of these old-field communities.
Soil microbial changes might also affect invasion by Cirsium, both directly and indirectly. Although the microbial community cultivated by Cirsium negatively affected at least some plant species, including the potential dominant Solidago canadensis, Cirsium’s own performance was not reduced by its associated soil organisms. This could lead to a competitive advantage for Cirsium through apparent as well as direct competition, consistent with models of disease-mediated invasion (Dunn et al. 2012). This study, therefore, contributes to the growing recognition of the potential importance of soil-mediated effects in plant invasions (Callaway et al. 2004; Mangla et al. 2008; Dickie et al. 2017).
References
Aleklett K, Leff JW, Fierer N, Hart M (2015) Wild plant species growing closely connected in a subalpine meadow host distinct root-associated bacterial communities. PeerJ 3:e804. https://doi.org/10.7717/peerj.804
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:612–619. https://doi.org/10.1007/s00442-004-1679-z
Balzergue C, Chabaud M, Barker DG, Bécard G, Rochange SF (2013) High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front Plant Sci 4:1–15. https://doi.org/10.3389/fpls.2013.00426
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511. https://doi.org/10.1038/nature13855
Bendall GM (1975) The allelopathic activity of Californian thistle [Cirsium arvense (L.) Scop.] in Tasmania. Weed Res 15:77–81
Berns AE, Philipp H, Narres HD, Burauel P, Vereecken H, Tappe W (2008) Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur J Soil Sci 59:540–550. https://doi.org/10.1111/j.1365-2389.2008.01016.x
Bever JD (1994) Feeback between plants and their soil communities in an old field community. Ecology 75:1965–1977
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol 157:465–473. https://doi.org/10.1046/j.1469-8137.2003.00714.x
Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: the utility of the feedback approach. Br Ecol Soc 85:561–573
Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol 66:265–283. https://doi.org/10.1146/annurev-micro-092611-150107
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, Van Der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J Ecol 94:893–904. https://doi.org/10.1111/j.1365-2745.2006.01158.x
Blaney CS, Kotanen PM (2001) Effects of fungal pathogens on seeds of native and exotic plants: a test using congeneric pairs. J Appl Ecol 38:1104–1113. https://doi.org/10.1046/j.1365-2664.2001.00663.x
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733. https://doi.org/10.1038/nature02322
Crawley MJ (2000) Seeds: the ecology of regeneration in plant communities, 2nd edn. CABI International, Wallingford
Cripps MG, Gassmann A, Fowler SV, Bourdôt GW, McClay AS, Edwards GR (2011) Classical biological control of Cirsium arvense: lessons from the past. Biol Control 57:165–174. https://doi.org/10.1016/j.biocontrol.2011.03.011
Crocker EV, Lanzafane JJ, Karp MA, Nelson EB (2016) Overwintering seeds as reservoirs for seedling pathogens of wetland plant species. Ecosphere 7:1–14. https://doi.org/10.1002/ecs2.1281
de la Peña E, de Clercq N, Bonte D, Roiloa S, Rodríguez-Echeverría S, Freitas H (2010) Plant-soil feedback as a mechanism of invasion by Carpobrotus edulis. Biol Invasions 12:3637–3648. https://doi.org/10.1007/s10530-010-9756-1
Del Fabbro C, Prati D (2015) Invasive plant species do not create more negative soil conditions for other plants than natives. Perspect Plant Ecol Evol Syst 17:87–95. https://doi.org/10.1016/j.ppees.2015.02.002
Dickie IA, St John MG, Yeates GW, Morse CW, Bonner KI, Orwin K, Peltzer DA (2014) Belowground legacies of Pinus contorta invasion and removal result in multiple mechanisms of invasional meltdown. AoB Plants. https://doi.org/10.1093/aobpla/plu056
Dickie IA, Bufford JL, Cobb RC, Desprez-Loustau ML, Grelet G, Hulme PE, Klironomos J, Makiola A, Nuñez MA, Pringle A, Thrall PH, Tourtellot SG, Waller L, Williams NM (2017) The emerging science of linked plant–fungal invasions. New Phytol 215:1314–1332. https://doi.org/10.1111/nph.14657
Diez JM, Dickie I, Edwards G, Hulme PE, Sullivan JJ, Duncan RP (2010) Negative soil feedbacks accumulate over time for non-native plant species. Ecol Lett 13:803–809. https://doi.org/10.1111/j.1461-0248.2010.01474.x
Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM, Byers JE, Coon CAC, Frankel VM, Holt RD, Hufbauer RA, Kanarek AR, Schierenbeck KA, Wolfe LM, Perkins SE (2012) Indirect effects of parasites in invasions. Funct Ecol 26:1262–1274. https://doi.org/10.1111/j.1365-2435.2012.02041.x
Engelkes T, Morriën E, Verhoeven KJFK, Bezemer TM, Biere A, Harvey JA, McIntyre LM, Tamis WLM, Van der Putten WH (2008) Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456:946–948. https://doi.org/10.1038/nature07474
Eppinga MB, Rietkerk M, Dekker SC, De Ruiter PC (2006) Accumulation of local pathogens: a new hypothesis to explain exotic plant invasions. Oikos 114:168–176
Fairbairn CB, Thomas B (1959) The potential nutritive value of some weeds common to north-eastern England. J Br Grassl Soc 14:36–46
Gibbons SM, Lekberg Y, Mummey DL, Sangwan N, Ramsey PW, Gilbert JA (2017) Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems 2:e00178-16. https://doi.org/10.1128/msystems.00178-16
Grove S, Haubensak KA, Gehring C, Parker IM (2017) Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. J Ecol 105:1496–1508. https://doi.org/10.1111/1365-2745.12853
Guggisberg A, Welk E, Sforza R, Horvath DP, Anderson JV, Foley ME, Rieseberg LH (2012) Invasion history of North American Canada thistle, Cirsium arvense. J Biogeogr 39:1919–1931. https://doi.org/10.1111/j.1365-2699.2012.02746.x
Gupta A (2010) Management of fungal plant pathogens. CABI International, Oxfordshire
Hale AN, Tonsor SJ, Kalisz S (2011) Testing the mutualism disruption hypothesis: physiological mechanisms for invasion of intact perennial plant communities. Ecosphere 2:art110. https://doi.org/10.1890/es11-00136.1
Harper JL (1977) Population biology of plants. Academic Press, London
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. University Press of Hawaii, Honolulu
Inderjit, van der Putten WH (2010) Impacts of soil microbial communities on exotic plant invasions. Trends Ecol Evol 25:512–519. https://doi.org/10.1016/j.tree.2010.06.006
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585. https://doi.org/10.1046/j.1469-8137.1997.00729.x
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70. https://doi.org/10.1038/417067a
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kotanen PM (2007) Effects of fungal seed pathogens under conspecific and heterospecific trees in a temperate forest. Can J Bot 85:918–925. https://doi.org/10.1139/B07-088
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant-soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992. https://doi.org/10.1111/j.1461-0248.2008.01209.x
Kulmatiski A, Beard KH, Grenzer J, Forero L, Heavilin J (2016) Using plant-soil feedback to predict plant biomass in diverse communities. Ecology 97:2064–2073. https://doi.org/10.1017/CBO9781107415324.004
Lebeis SL (2015) Greater than the sum of their parts: characterizing plant microbiomes at the community-level. Curr Opin Plant Biol 24:82–86. https://doi.org/10.1016/j.pbi.2015.02.004
Li H, Zhang X, Zheng R, Li X, Elmer WH, Wolfe LM, Li B (2014) Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native saltmarsh plants in China. J Ecol 102:1112–1119. https://doi.org/10.1111/1365-2745.12285
Locket GH (1946) Observations on the colonization of bare chalk. J Ecol 33:205–209
MacKay J, Kotanen PM (2008) Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area. J Ecol 96:1152–1161. https://doi.org/10.1111/j.1365-2745.2008.01426.x
Mangla S, Inderjit, Callaway RM (2008) Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 96:58–67. https://doi.org/10.1111/j.1365-2745.2007.01312.x
Meng L-H, Wang Y, Luo J, Yang Y-P, Duan YW (2014) The trade-off and altitudinal variations in seed weight-number in Sinopodophyllum hexandrum (Royle) Ying (Berberidaceae) populations from the Hengduan Mountains. Pol J Ecol 62:413–419
Moeller HV, Dickie IA, Peltzer DA, Fukami T (2015) Mycorrhizal co-invasion and novel interactions depend on neighborhood context. Ecology 96:2336–2347. https://doi.org/10.1890/14-2361.1
Moore RJ (1975) The biology of Canadian weeds: Cirsium arvense (L.) Scop. Can J Plant Sci 55:1033–1048
Nijjer S, Rogers WE, Siemann E (2007) Negative plant-soil feedbacks may limit persistence of an invasive tree due to rapid accumulation of soil pathogens. Proc R Soc B 274:2621–2627. https://doi.org/10.1098/rspb.2007.0804
Nunes KA, Kotanen PM (2018) Comparative impacts of aboveground and belowground enemies on an invasive thistle. Ecol Evol 8:1430–1440. https://doi.org/10.1002/ece3.3751
O’Hanlon-Manners DL, Kotanen PM (2004) Evidence that fungal pathogens inhibit recruitment of a shade-intolerant tree, white birch (Betula papyrifera), in understory habitats. Oecologia 140:650–653. https://doi.org/10.1007/s00442-004-1625-0
Orrock JL, Christopher CC, Dutra HP (2012) Seed bank survival of an invasive species, but not of two native species, declines with invasion. Oecologia 168:1103–1110. https://doi.org/10.1007/S00442-01
Packer A, Clay K (2003) Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology 84:108–119. https://doi.org/10.1890/0012-9658(2003)084%5b0108:SPAPSS%5d2.0.CO;2
Parker IM, Saunders M, Bontrager M, Weitz AP, Hendricks R, Magarey R, Suiter K, Gilbert GS (2015) Phylogenetic structure and host abundance drive disease pressure in communities. Nature 520:542–552. https://doi.org/10.1038/nature14372
Pernilla Brinkman E, Van der Putten WH, Bakker E-J, Verhoeven KJF (2010) Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J Ecol 98:1063–1073. https://doi.org/10.1111/j.1365-2745.2010.01695.x
Preston DL, Mischler JA, Townsend AR, Johnson PTJ (2016) Disease ecology meets ecosystem science. Ecosystems 19:737–748. https://doi.org/10.1007/s10021-016-9965-2
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen HN, Dixon KW, Jersáková J, Těšitelová T (2015) Germination and seedling establishment in orchids: a complex of requirements. Ann Bot 116:391–402. https://doi.org/10.1093/aob/mcv087
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmanek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93. https://doi.org/10.1111/j.1469-185X.1999.tb00041.x
Rodríguez-Echeverría S, Crisóstomo JA, Nabais C, Freitas H (2009) Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions 11:651–661. https://doi.org/10.1007/s10530-008-9280-8
Schafer M, Kotanen PM (2004) Impacts of naturally-occurring soil fungi on seeds of meadow plants. Plant Ecol 175:19–35. https://doi.org/10.1023/B:VEGE.0000048096.00772.23
Schroeder MS, Janos DP (2004) Phosphorus and intraspecific density alter plant responses to arbuscular mycorrhizas. Plant Soil 264:335–348
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4:727–731. https://doi.org/10.1371/journal.pbio.0040140
Templeton AR, Levin DA (1979) Evolutionary consequences of seed pools. Am Nat 114:232–249
Tiley GED (2010) Biological flora of the British Isles: Cirsium arvense (L.) Scop. J Ecol 98:938–983. https://doi.org/10.1111/j.1365-2745.2010.01678.x
Torgeson DC (1969) Fungicides: an advanced treatise. Academic Press, New York
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
van der Putten WH, Peters BAM (1997) How soil-borne pathogens may affect plant competition. Ecology 78:1785–1795
van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53–56. https://doi.org/10.1038/362053a0
van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37. https://doi.org/10.1038/ismej.2007.9
van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Van de Voorde TFJ, Wardle DA (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276
van Grunsven RHA, van der Putten WH, Bezemer TM, Tamis WLM, Berendse F, Veenendaal EM (2007) Reduced plant-soil feedback of plant species expanding their range as compared to natives. J Ecol 95:1050–1057
Vandegehuchte ML, de la Peña E, Bonte D (2010) Relative importance of biotic and abiotic soil components to plant growth and insect herbivore population dynamics. PLoS One 5:1–8. https://doi.org/10.1371/journal.pone.0012937
Wilson RG (1981) Effect of Canada thistle (Cirsium arvense) residue on growth of some crops. Weed Sci 29:159–164
Wolfe BE, Klironomos JN (2005) Breaking new ground: soil communities and exotic plant invasion. Bioscience 55:477–487. https://doi.org/10.1641/0006-3568(2005)055%5b0477:bngsca%5d2.0.co;2
Acknowledgements
This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (PMK). We thank K. Nunes for her advice and help in the lab; A. Longley for her help in the field and lab; S. Shukla and R. Matar for their work in the greenhouse and with weighing; S. Schneider and all the KSR staff for ensuring that field experiments ran smoothly; two anonymous reviewers who provided comments to improve the manuscript. This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Contributions
PMK conceived the initial idea, and JDV and PMK designed the experiments. JDV conducted the experiments and analysis of data, and PMK provided guidance. JDV wrote the manuscript, and PMK provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Data availability
Upon acceptance of the manuscript, data will be archived in the Dryad Digital Repository.
Additional information
Communicated by Edith B. Allen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verbeek, J.D., Kotanen, P.M. Soil-mediated impacts of an invasive thistle inhibit the recruitment of certain native plants. Oecologia 190, 619–628 (2019). https://doi.org/10.1007/s00442-019-04435-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04435-8