Abstract
Current declines in the abundance and diversity of bees and other pollinators has created uncertainty in their ability to reliably deliver pollination services. Recent studies examining urban bee diversity have provided conflicting results, with some studies identifying parts of cities with high bee diversity and others documenting reduced diversity with high levels of urbanization, with potential effects on surrounding agricultural areas. However, these studies have not specifically investigated pollination services, or examined the influence of local habitat conditions on these services. We surveyed urban gardens and city parks across the metropolitan region of Toledo, Ohio (USA) to understand how urbanization (impervious surface) and local habitat characteristics (herbaceous cover, floral abundance and color, tree abundance, canopy cover, soil moisture, garden size) impact bee communities (abundance, diversity, composition) and pollination services (visitation frequency). We collected 729 bees representing 19 genera and 57 species. We found that bee community composition was strongly associated with percent impervious surface. Bee abundance declined with increased canopy cover and impervious surface, while declines in bee diversity with increasing impervious surface were greatly reduced by increases in floral resources. Visitation rates were positively correlated with bee abundance and diversity, declining with increased impervious surface, but increasing with floral resource availability. These results suggest that increasing floral resources at high impervious sites may counteract the negative effects of impervious surface on bee diversity and pollination services in cities similar to Toledo, OH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global significance of pollinators has been well established—bees and other pollinating animals provide important pollination services that benefit ~ 87% of flowering plants (angiosperms) worldwide (Ollerton et al. 2011), including 1500 agricultural crops (Klein et al. 2007). Pollination can increase the quality, quantity, and stability of agricultural yields (Allen-Wardell et al. 1998; Ricketts et al. 2008), and the estimated global value of pollination services is $117 billion annually (Costanza et al. 1997). However, there is strong evidence that wild and managed pollinators are in decline globally (Potts et al. 2010), and anthropogenic modification of natural landscapes via habitat loss, fragmentation, and land use intensification is a primary driver behind these declines (Ricketts et al. 2008; Potts et al. 2010). But evaluating how anthropogenic landscape modification affects pollination can be difficult to assess, because pollination is influenced by a myriad of environmental conditions that vary across spatial scales.
In general, pollination services are strongly associated with the availability of floral and nesting resources. Multiple studies have found positive effects of floral resource availability on pollination (Kells et al. 2001; Blaauw and Isaacs 2014). For instance, Blaauw and Isaacs (2014) found that increased wildflower abundances near crop fields improved pollination and crop yields, and even lead to profit gains. Nesting resource availability (e.g., bare ground, pre-existing cavities) also influences pollination through changes in bee community structure (Potts et al. 2005). Others have found declines in pollination with increased distance from natural areas (Ricketts et al. 2008). Land-use intensification (e.g., urbanization) may also influence pollination, but few have investigated pollination across intensification gradients outside of traditional agricultural systems. Much of our understanding of pollination comes from traditional agriculture, but urban agriculture is an increasingly important sector of the global food supply (Hodgson et al. 2011).
Currently, over half of the global human population lives in urban regions (Pickett et al. 2011), and over 80% of the United States population is considered urban (United Nations 2018). The amount of terrestrial land classified as urban is expected to triple by 2030 (Seto et al. 2012), transforming rural regions into residences and infrastructure for an increasingly urban human population. The overall impact urbanization has on species and ecosystem services is difficult to assess because there is a great deal of variation in the spatial heterogeneity and development intensity within and among cities (Lin and Fuller 2013), and between shrinking and growing cities (Haase 2008). But a number of studies over the past decade have shown that bee community responses to urbanization are often mediated by local and landscape habitat conditions (Ahrne et al. 2009; Hernandez et al. 2009; Fortel et al. 2014; Quistberg et al. 2016; Glaum et al. 2017; Hall et al. 2017). Floral resource availability is consistently found to be a strong predictor of bee abundance (Lowenstein et al. 2014; Pardee and Philpott 2014) and diversity (Lowenstein et al. 2014; Pardee and Philpott 2014) in cities. Quistberg et al. (2016) found that larger urban greenspaces harbor more bee individuals and species, and that ground cover (e.g., mulch, leaflitter) influenced the types of bees present (e.g., cavity nesting taxa). But few studies simultaneously consider the effects of urbanization and local habitat features on both diversity and pollination services (but see Potter and Lebuhn 2015).
Despite the importance of pollination in urban greenspaces (e.g., city parks and urban gardens), surprisingly few studies have explicitly measured pollination services in cities (Lowenstein et al. 2014, 2015; Theodorou et al. 2016). Lowenstein et al. (2014) measured pollination services in residential yards in Chicago (USA), and found a positive correlation of bee abundance and diversity on visitation frequency, but they did not examine drivers of these patterns and did not examine highly urbanized parts of the city. Others have also identified the positive effect bee diversity has on fruit set in pollinator-dependent plants (Kremen et al. 2002), suggesting a direct relationship between bee diversity and pollination. However, the factors that drive bee diversity are not always directly associated with pollination. For instance, Lowenstein et al. (2014) found a positive association of floral richness with bee diversity, but not pollination (e.g., visitation frequency). Thus, additional studies are needed to investigate how local habitat characteristics influence both bee diversity and pollination across urbanization gradients in cities.
In this study, we investigated how habitat characteristics (herbaceous cover, floral abundance and color, tree abundance, canopy cover, soil moisture, garden size) in city parks and urban gardens influenced the abundance, diversity, and community composition of bees, and the visitation frequency of insect pollinators. We divided this overarching question into two parts: (1) How does urbanization influence bee communities (abundance, diversity, composition) and pollination services (visitation frequency)? (2) How do local habitat features within urban gardens and city parks influence or alter the effects urbanization has on bee communities and visitation frequencies? We expected to see changes in bee community composition with urbanization, with concomitant declines in abundance and diversity, likely due to changes in the availability and quality of habitat (e.g., highly urban areas have less greenspace and are hotter). We also predicted positive correlations between bee abundance and flower abundance, due to increased resource availability. But we were uncertain whether positive effects of floral resources would be sufficient to counteract the negative effects of impervious surface or of the relative importance of other local habitat factors on pollination services.
Methods
Sampling location
We sampled bees from a total of 30 sites (parks and gardens) in the metropolitan region of Toledo, OH, USA (Fig. 1). This 620-km2 region is home to a half-million people, and its network of over 150 community gardens and 125 city parks was utilized for sampling locations (Burdine and Taylor 2017). We selected our 30 sites by overlaying a grid across a map of metropolitan Toledo in ArcGIS, and each grid cell (2 km × 2 km) was numbered. We then used a random number generator to select which grid cells to include in the study, and within each of those selected grid cells, we identified a single park or garden to sample using a random number generator. Parks or gardens ranged in size from 0.001 to 0.46 km2.
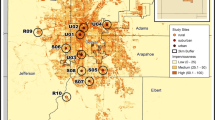
Map of study sites overlaid with percent impervious surface in Toledo, Ohio, USA. Impervious surface was calculated within a 300-m radius around each site. Regions of dark red are high impervious surface, and lighter shades of red are low impervious surface. Map constructed using data from the 2011 NLCD Percent Developed Impervious layer (Homer et al. 2015)
Sampling methods
We collected bees using elevated pan traps once per month between June and August in 2016. Sampling was restricted to sunny days with temperatures above 22 °C. We constructed the elevated pan traps by placing a 175-ml plastic bowl (yellow, blue, or white) atop 1-m PVC pipe (Tuell and Isaacs 2009). Bowls were painted with Krylon ColorMaster® spray paint to enhance visibility, and each bowl was filled with a water and soap mixture. On sampling days, we placed 9 elevated pan traps (three yellow, three blue, three white) along a transect at the center of each site and left them in the field for 24 h. During collection, the contents of all nine pan traps were combined into a single container for transport to the lab, and in the lab, bees were separated from the other bycatch insects. Once sorted, bees were preserved in ethanol prior to pinning, and identified to species or morphospecies. We identified species using a synoptic collection from Pardee and Philpott (2014), and the Discover Life bee species guides (Ascher and Pickering 2016).
Local habitat characteristics
We measured local habitat characteristics at each site during sampling events. All characteristics were measured with the center of each site as the focal point, corresponding to the pan trap locations. We calculated canopy cover when facing each cardinal direction away from the site’s center using a densiometer. We also counted the number of trees within 25 m of the site’s center. Additionally, we walked a 10-m transect out from the center of each site and counted the total number of flowers in bloom within 1 m of the transect line, and recorded their color. We measured floral color as a predictor as others have done (Pardee and Philpott 2014; Quistberg et al. 2016), since bees can have preferences for specific flower colors (Campbell et al. 2010). We calculated groundcover by randomly placing four quadrats (1 m × 1 m) along each transect, and estimated the percentage of herbaceous vegetation, woody vegetation, and bare ground cover (similar to Lagucki et al. 2017). We also took four measurements of volumetric soil moisture along the transect using a soil moisture meter (Delta-T Devices SM150), as a proxy for water availability. Water drinking behavior has been commonly observed in honeybees, and there is evidence that honeybees may be vulnerable to desiccation in cities (Burdine and McCluney 2019). Additionally, extremely high soil moisture levels may adversely impact certain bees (e.g., ground nesters). Thus, we focused on highly localized factors (within 25 m) that could influence bee abundance, diversity, community composition, and pollination given variation in the degree of urbanization surrounding each garden or park.
To assess the urbanization of the surrounding landscape, we estimated percent impervious surface within a 300-m radius of each site’s center, using the National Land Cover Databases’ dataset for 2011 Percent Development Imperviousness (Homer et al. 2015).
Visitation rates
We estimated pollinator visitation rates at the center of each site, during each sampling event, by placing five flowering plants: (1) tomato (early girl variety), (2) purple headed cone flower (Echinacea purpurea), (3) brown-eyed susans (Rudbeckia triloba), (4) bergamot (Monarda fistulosa), and (5) foxglove (Penstemon digitalis). We selected these five plants because they are attractive to pollinators and are commonly found in Toledo parks and gardens (Pardee and Philpott 2014; Burdine and Taylor 2017). We counted the total number of individual insect pollinators that visited the plants over a 20-min timespan (similar to Lowenstein et al. 2014). We used these measures to calculate a visitation rate for each site (visits/hour), which others found to be well correlated with fruit production (Garibaldi et al. 2013).
Statistical methods
We conducted all statistical tests using the program R. The cor function in R was used to examine collinearity between our environmental factors; for co-correlated factors (R > 0.5), we dropped one of the factors from statistical analyses (Table S5). We used this cut-off for the level of correlation to constrain the potential model set. We examined the effects of environmental factors on community composition with a Type II PERMANOVA (adonis.II) using the “RVAideMemoire” package. We also utilized non-metric multidimensional scaling (metaMDS) within the “vegan” package to display differences in community composition, and associations with environmental factors. We used Bray–Curtis distances for all community composition analyses.
We used generalized linear models (glm) to examine relationships among environmental factors and dependent variables (abundance, diversity, visitation frequency). Prior to statistical analysis, we combined the three sampling periods (June, July, August) by totaling the number of bees captured. Models were developed by first establishing a list of candidate models that contained each potential predictor independently (excluding co-correlated variables, Table S1). Then, we took the model(s) with the lowest AIC values (models within two AIC units were considered equivalent) and combined the models to test whether the combined model was a better fit (two AIC units lower). We chose this process instead of model averaging approaches because they can be problematic with interactive models (Cade 2015; Harrison et al. 2018). We also tested for interactions between various site-level environmental factors and impervious surface to examine potential modifiers of any potential urbanization effect. Assumptions of normality and equal variance were assessed by examining plots of residuals and data transformations were used when necessary. We tested for spatial autocorrelation using the “ape” package in R (Paradis et al. 2004), and our results showed no spatial autocorrelation in the dependent variables (Table S3). We also tested whether site type (urban garden vs. city park) had an impact on the dependent variables (abundance, diversity, visitation frequency), and found no significant differences (Table S4).
Results
Summary statistics
We collected a total of 729 bees representing 19 genera and 57 species from 30 sites. The majority of bees sampled were females (84.2%). The most common genera in order of abundance were the sweat bee Lasioglossum (48.4%), the long-horned bee Melissodes (8.8%), the striped sweat bee Agapostemon (7.57%), the mining bee Halictus (7.02%), and the sweat bee Augochlora (6.88%). The most diverse genera in order of number of species were the sweat bee Lasioglossum (13 species), the long-horned bee Melissodes (7 species), the bumblebee Bombus (5 species), and the leafcutter bee Megachile (5 species). Across sampling periods, we collected between 2 and 19 species per site.
Community Composition
We found impervious surface to be the only environmental variable significantly associated with bee community composition (PERMANOVA F1,21 = 1.99, p = 0.01; Table 1). Nonmetric dimensional scaling plots (Fig. 2b) indicated that Lasioglossum imitatum was positively associated with urbanization, and four species were negatively associated with urbanization: Bombus bimaculatus, Lasioglossum fatiggi, Hylaeus annulatus, and Hylaeus illinoisensis.
Nonmetric multidimensional scaling (NMDS) analysis for bee species sampled in Toledo, Ohio (USA). a Each bee species is represented by a single point, and all environmental factors are represented by arrows. b Impervious surface was the only environmental factor significantly associated with community composition. Specific taxa that were positively or negatively correlated with impervious surface are labeled
Overall abundance and diversity
We found several local factors to be strongly associated with the overall abundance and diversity of bees. The most parsimonious model for bee abundance was an additive model with abundance declining with increased canopy cover and impervious surface (AIC = 247.11; R2 = 0.27; Fig. 3a, b; Table 2). We identified two additional models with similar AIC values that were correlated with bee abundance. One model included the interaction of canopy cover and impervious surface (AIC = 248.31, R2 = 0.30, Fig. 4a; Table 2) and the second model included canopy cover, but not impervious surface (AIC = 248.37, R2 = 0.18, Fig. 3a; Table 2). For bee diversity, the most parsimonious model included the interaction of impervious surface and purple flower abundance (AIC = 31.96; R2 = 0.5; Fig. 4b; Table 2), with diversity declining with impervious surface, but only when purple flowers were not abundant. We identified an additional model with similar AIC that included the interaction of total flower abundance and impervious surface (AIC = 33.48; R2 = 0.48; Table 2), showing a similar pattern.
Panel figure displaying interaction plots. (a) Relationship between impervious surface and bee abundance when canopy cover is at a high (+ 1 SD), low (-1 SD), or medium level (mean). (b) Relationship between impervious surface and bee diversity when purple flower abundance is at a high (+ 1 SD), low (-1 SD), or medium level (mean)
Visitation rates
The most parsimonious model for visitation rates was an additive model with visitation declining with impervious surface, but increasing with flower abundance (AIC = 255.59; R2 = 0.67; Fig. 3c, d; Table 2), explaining 67% of the variation in visitation. In addition, we found a positive correlation between visitation rates and bee abundance (R = 0.49) and diversity (R = 0.44).
Discussion
Overall, our results indicate that bee diversity and pollination services decline with increased urbanization, but local habitat features can modify the effects of urbanization. More specifically, abundant flowers (all or purple) can help to prevent urbanization-related declines in diversity and pollination services. Although these results might have been expected from other research, mostly outside cities, showing positive effects of flowers (Kells et al. 2001; Blaauw and Isaacs 2014) on bee abundance, diversity, and pollination, they are in contrast with another recent study which does not indicate that flowers can rescue urban bees (Hamblin et al. 2018). Although there are many potential mechanisms underlying the differences observed between that study and ours, background climate may be one important factor. Hamblin et al. (2017) found that differences in thermal tolerance between species strongly drove abundance of bees along a gradient of urban-related warming in Raleigh, NC, an already warm southeastern city. This contrasts with a recent study finding that three species of bees in urban parts of Toledo, OH, a cooler city, are not near their thermal limits, and thus are unlikely to be influenced by urban-warming (Burdine and McCluney 2019). Thus, flowers may be unable to rescue bees from urban-warming in already warm climates, but may be sufficient to reduce declines in cooler cities. Other explanations are possible; in general, more work is needed to better identify regional differences in both the effects of urbanization and the potential for mitigation of urbanization via floral resources, or other factors. But here, we show that improved floral resources can mitigate urban-related declines in pollinators and pollination services in Toledo, OH.
Impervious surface
Impervious surface was the only habitat characteristic associated with community composition. In particular, we found a positive association of impervious surface on Lasioglossum imitatum, a solitary and ground nesting species. Normandin et al. (2017) provide evidence that this species can be abundant in certain urban habitats (e.g., cemeteries). On the other hand, we identified 11 species that were exclusively present at low impervious sites (< 25%), and one species present only at high impervious sites (> 50%). Multiple studies have identified changes in bee community composition across urbanization gradients (Bates et al. 2011; Fortel et al. 2014), and degraded nest site availability with increasing impervious surface may explain changes in composition (Cane et al. 2006).
Canopy cover
We found a negative association between canopy cover and bee abundance, and the association was stronger at low impervious sites. Others have identified canopy cover as a significant predictor of bee abundance in non-urban systems (Jha and Vandermeer 2010), and particularly for solitary species. There are examples of other arthropod taxa responding negatively to canopy cover in cities (Philpott et al. 2014; Lagucki et al. 2017). Matteson and Langellotto (2010) found that shading from buildings in New York City reduced sunlight availability in urban greenspaces, negatively impacting species richness of bees. Increased shade may prevent bees from maintaining optimal body temperatures by passive basking in greenspaces (Matteson and Langellotto 2010), and this may explain why canopy cover associations were stronger at low impervious sites (reduced heat island effects). Increased shade in urban regions can also reduce floral abundance, and Matteson et al. (2013) show that this can indirectly impact insect pollinators.
Flower abundance
Others have identified the importance of floral availability in maintaining diverse bee assemblages in cities (Matteson and Langellotto 2010; Pardee and Philpott 2014; Quistberg et al. 2016), but here we find this pattern occurs across sites with both high and low impervious surface, with flowers restricting declines in bee diversity and pollination that would otherwise be seen in cities. Lowenstein et al. (2014) shows that increased floral diversity can mitigate any potential negative effects of urbanization, even in densely populated regions, and visitation frequencies may even increase with urbanization. Potter and LeBuhn (2015) also identified positive correlations between floral resource density and pollination services across urban garden sites. However, increasing floral resources is less effective in warmer cities like Raleigh, NC (Hamblin et al. 2018). We expected to find a positive relationship between flower abundance and visitation frequencies, and the strength of the relationship (R2 = 0.57) suggests that increased flower availability might strongly help to prevent declines in pollination services at high impervious sites.
Caveats
Our research has several methodological limitations. First, by only using pan traps to sample bees we may have under-sampled certain taxa. Others have shown that pan traps can underrepresent larger bees (Roulston et al. 2007), but we still collected many large bees (e.g., honeybees, bumblebees) and this method of capture was constant across all sampling sites, providing robust metrics of relative differences between sites. Second, visitation rates may not always reflect pollination. Visitors are not necessarily pollinating flowers, and others have suggested combining measures of visitation with an estimate of pollinator effectiveness (King et al. 2013). However, there are instances within agricultural systems, such as those studied here, where visitation rates have been shown to be a good metric of pollination (Garibaldi et al. 2013), but more work is needed in urban agricultural systems. Third, we included both urban gardens and city parks as samping sites because both are greenspaces embedded within urban landscapes. Even though these site types are different types of greenspaces, we did not detect different effects based on site type (see Table S4).
Conclusions
We show that negative effects of urbanization on bee communities and pollination services can be altered by local habitat characteristics (flower abundance, canopy cover). More specifically, increasing the total number of flowers could be an important strategy for improving pollination services, independent of whether the garden is embedded within a highly impervious habitat.
References
Ahrne K, Bengtsson J, Elmqvist T (2009) Bumble bees (Bombus spp) along a gradient of increasing urbanization. PLoS One 4:e5574. https://doi.org/10.1371/journal.pone.0005574
Allen-Wardell G, Bernhardt P, Bitner R et al (1998) The potential consequences of pollinator declines on the conservatoin of biodiversity and stability of food crop yields. Conserv Biol 12:8–17
Ascher JS, Pickering J (2016) Discover life bee species guide and world checklist (Hymenoptera: Apoida: Anthopila). http://www.discoverlife.org/mp/20q?guide=Apoidea_species. Accessed 15 Sept 2017
Bates AJ, Sadler JP, Fairbrass AJ et al (2011) Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS One 6:e23459. https://doi.org/10.1371/journal.pone.0023459
Blaauw BR, Isaacs R (2014) Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J Appl Ecol 51:890–898. https://doi.org/10.1111/1365-2664.12257
Burdine JD, McCluney KE (2019) Differential sensitivity of bees to urbanization-driven changes in body temperature and water content. Sci Rep 9:1643. https://doi.org/10.1038/s41598-018-38338-0
Burdine JD, Taylor DE (2017) Neighbourhood characteristics and urban gardens in the Toledo metropolitan area: staffing and voluntarism, food production, infrastructure, and sustainability practices. Local Environ 23:198–219. https://doi.org/10.1080/13549839.2017.1397614
Cade BS (2015) Model averaging and muddled multimodel inferences. Ecology 96:2370–2382. https://doi.org/10.1890/14-1639.1
Campbell DR, Bischoff M, Lord JM et al (2010) Flower color influences insect visitation in alpine New Zealand. Ecology 91:2638–2649. https://doi.org/10.1890/09-0941.1
Cane JH, Minckley RL, Kervin LJ et al (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol Appl 16:632–644. https://doi.org/10.1890/1051-0761(2006)016[0632:CRWADB]2.0.CO;2
Costanza R, Arge R, De Groot R et al (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260. https://doi.org/10.1038/387253a0
Fortel L, Henry M, Guilbaud L et al (2014) Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 9:e104679. https://doi.org/10.1371/journal.pone.0104679
Garibaldi LA, Steffan-Dewenter I, Winfree R et al (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611. https://doi.org/10.1126/science.1230200
Glaum P, Simao M, Vaidya C et al (2017) Big city Bombus: using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. R Soc Open Sci 4:170156. https://doi.org/10.1098/rsos.170156
Haase D (2008) Urban ecology of shrinking cities: an unrecognized opportunity? Nat Cult 3:1–8. https://doi.org/10.3167/nc.2008.030101
Hall D, Camilo G, Tonietto et al (2017) The city as a refuge for insect pollinators. Conserv Biol 31:24–29. https://doi.org/10.1111/cobi.12840
Hamblin AL, Youngsteadt E, Lopez-Uribe MM et al (2017) Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biol Lett 13:20170125. https://doi.org/10.1098/rsbl.2017.0125
Hamblin AL, Youngsteadt E, Frank SD (2018) Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst 21:419–428. https://doi.org/10.1007/s11252-018-0731-4
Harrison XA, Donaldson L, Correa-Cano ME et al (2018) A brief introduction to mixed effects modelling and multi-model infernece in ecology. PeerJ 6:e4794. https://doi.org/10.7717/peerj.4794
Hernandez JL, Frankie GW, Thorp RW (2009) Ecology of urban bees: a review of current knowledge and directions for future study. Cities Environ 2:1–15
Hodgson K, Campbell MC, Bailkey M (2011) Urban agriculture: growing healthy, sustainable places. Planning Advisory Service Report Number 563. American Planning Association, Washington, DC
Homer CG, Dewitz JA, Yang L et al (2015) Completion of the 2011 National Land Cover Database for the conterminous United States—representing a decade of land cover change information. Photogramm Eng Remote Sens 81:345–354. https://doi.org/10.14358/PERS.81.5.345
Jha S, Vandermeer JH (2010) Impacts of coffee agroforestry management on tropical bee communities. Biol Conserv 143:1423–1431. https://doi.org/10.1016/j.biocon.2010.03.017
Kells AR, Holland JM, Goulson D (2001) The value of uncropped field margins for foraging bumblebees. J Insect Conserv 5:283–291. https://doi.org/10.1023/A:1013307822575
King C, Ballantyne G, Willmer PG (2013) Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods Ecol Evol 4:811–818. https://doi.org/10.1111/2041-210X.12074
Klein A-M, Vaissière BE, Cane JH et al (2007) Importance of pollinators in changing landscapes for world crops. Proc Biol Sci 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA 99:16812–16816. https://doi.org/10.1073/pnas.262413599
Lagucki E, Burdine JD, McCluney KE (2017) Urbanization alters communities of flying arthropods in parks and gardens of a medium-sized city. PeerJ 5:e3620. https://doi.org/10.7717/peerj.3620
Lin BB, Fuller RA (2013) Sharing or sparing? How should we grow the world’s cities? J Appl Ecol 50:1161–1168. https://doi.org/10.1111/1365-2664.12118
Lowenstein DM, Matteson KC, Xiao I et al (2014) Humans, bees, and pollination services in the city: the case of Chicago, IL (USA). Biodivers Conserv 23:2857–2874. https://doi.org/10.1007/s10531-014-0752-0
Lowenstein DM, Matteson KC, Minor ES (2015) Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 179:811–821. https://doi.org/10.1007/s00442-015-3389-0
Matteson K, Langellotto GA (2010) Determinates of inner city butterfly and bee species richness. Urban Ecosyst 13:333–347. https://doi.org/10.1007/s11252-010-0122-y
Matteson KC, Grace JB, Minor ES (2013) Direct and indirect effects of land use on floral resources and flower-visiting insects across an urban landscape. Oikos 122:682–694. https://doi.org/10.1111/j.1600-0706.2012.20229.x
Normandin É, Vereecken NJ, Buddle CM, Fournier V (2017) Taxonomic and functional trait diversity of wild bees in different urban settings. Peer J 5:e3051. https://doi.org/10.7717/peerj.3051
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. https://doi.org/10.1093/bioinformatics/btg412
Pardee GL, Philpott SM (2014) Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosyst 17:641–659. https://doi.org/10.1007/s11252-014-0349-0
Philpott SM, Cotton J, Bichier P et al (2014) Local and landscape drivers of arthropod abundance, richness, and trophic composition in urban habitats. Urban Ecosyst 17:513–532. https://doi.org/10.1007/s11252-013-0333-0
Pickett STA, Cadenasso ML, Grove JM et al (2011) Urban ecological systems: scientific foundations and a decade of progress. J Environ Manage 92:331–362. https://doi.org/10.1016/j.jenvman.2010.08.022
Potter A, LeBuhn G (2015) Pollination service to urban agriculture in San Francisco, CA. Urban Ecosyst 18:885–893. https://doi.org/10.1007/s11252-015-0435-y
Potts SG, Vulliamy B, Roberts S et al (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol Entomol 30:78–85. https://doi.org/10.1111/j.0307-6946.2005.00662.x
Potts SG, Biesmeijer JC, Kremen C et al (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Quistberg RD, Bichier P, Philpott SM (2016) Landscape and local correlates of bee abundance and species richness in urban gardens. Environ Entomol 45:592–601. https://doi.org/10.1093/ee/nvw025
Ricketts T, Regetz J, Steffan-Dewenter Cunningham SA et al (2008) Landscape effects on crop pollination services: are there general patterns? Ecol Lett. https://doi.org/10.1111/j.1461-0248.2008.01157.x
Roulston TA, Smith SA, Brewster AL (2007) A comparison of pan trap and intensive net sampling technique for documenting a bee (Hymenoptera: Apiformes) fauna. J Kansas Entomol Soc 80:179–181. https://doi.org/10.2317/0022-8567(2007)80%5b179:ACOPTA%5d2.0.CO;2
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1211658109
Theodorou P, Radzevičiūtė R, Settele J et al (2016) Pollination services enhanced with urbanization despite increasing pollinator parasitism. Proc R Soc B Biol Sci 283:20160561. https://doi.org/10.1098/rspb.2016.0561
Tuell JK, Isaacs R (2009) Elevated pan traps to monitor bees in flowering crop canopies. Entomol Exp Appl 131:93–98. https://doi.org/10.1111/j.1570-7458.2009.00826.x
United Nations (2018) 2018 Revision of World Urbanization Prospects (United Nations: New York). Available at: https://population.un.org/wup/. Accessed 1 Sept 2018
Acknowledgements
We thank the following organization and entities for giving us permission to collect bees on their properties: Toledo Botanical Gardens, Toledo Grows, MultiFaith Grows, Toledo City Parks, Olander Park System, Toledo Zoo, Wood County Parks, and the City of Holland. We also thank Bowling Green State University for providing funding. Edward Lagucki and Erin Plummer assisted in sorting, cleaning, and pinning our bee specimens. We also thank Andrew Gregory, Helen Michaels, Shannon Pelini, and Elsa Youngsteadt for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
JDB and KEM conceived and designed the study. JDB executed the study and wrote the manuscript. KEM edited the manuscript.
Corresponding author
Additional information
Communicated by Nina Farwig.
Urbanization-related declines in bee diversity and flower visitation may be mediated by local habitat features. Floral resources reduced declines even at highly urbanized sites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burdine, J.D., McCluney, K.E. Interactive effects of urbanization and local habitat characteristics influence bee communities and flower visitation rates. Oecologia 190, 715–723 (2019). https://doi.org/10.1007/s00442-019-04416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04416-x