Abstract
Precipitation changes may induce shifts in plant species or life form dominance in ecosystems, making some previously subordinate species abundant. The plasticity of certain plant functional traits of these expanding subordinate species may be one possible mechanism behind their success. In this study, we tested if the subordinate winter annual grass Secale sylvestre shows plasticity in growth and reproduction in response to altered environment associated with field-scale rainfall manipulations (severe drought, moderate drought, and watering) in a semiarid grassland, and whether the maternal environment influences offspring germination or growth in a subsequent pot experiment. Compared to control plots, S. sylvestre plants grew 38% taller, and produced 32% more seeds in severe drought plots, while plants in watered plots were 17% shorter, and had 22% less seeds. Seed mass was greatest in severe drought plots. Plants growing in drought plots had offspring with enhanced juvenile shoot growth compared to the progeny whose mother plants grew in watered plots. These responses are most likely explained by the decreased cover of previously dominant perennial grasses in severe drought plots, which resulted in wetter soil compared to control and watered plots during the peak growth of S. sylvestre. We conclude that the plasticity of this subordinate annual species in response to changing environment may help to gain dominance with recurring droughts that suppress perennial grasses. Our results highlight that exploring both within-generation and transgenerational plasticity of subordinate species may lead to a better prediction of changes in plant species dominance under climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In arid and semiarid grasslands, water availability is a strong determinant of plant diversity, primary production, and community stability (Sala et al. 1988; Bai et al. 2004; Suttle et al. 2007; Seddon et al. 2016). In these ecosystems, altered precipitation regimes can often result in shifts in functional group abundances, species reordering, or even replacement of species within a community (Suttle et al. 2007; Smith et al. 2009; Scott et al. 2010; Dudney et al. 2017). In such cases, altered conditions may favour coexisting subordinate or transient species at the expense of the previous dominants (Mariotte et al. 2013; Yang et al. 2016). The identification of mechanisms at the level of functional group or individual species underlying these marked vegetation changes can be important to better understand and predict the impacts of climate change.
Plant functional traits of subordinate species have received relatively little attention compared to dominant species, despite the evidence that subordinates can also play a substantial role in maintaining ecosystem functions under stress (Walker et al. 1999; Mariotte et al. 2013; Mariotte 2014). Furthermore, the fact that subordinate species are often impacted indirectly by altered climatic conditions via changes in competitive interactions with the dominant species (Kardol et al. 2010; Mariotte et al. 2013, but see Levine et al. 2010) can make their response more difficult to forecast. This highlights the need to improve our understanding of how traits of subordinate species respond to altered climate change drivers, such as precipitation.
Phenotypic plasticity is one of the key mechanisms—besides shifts in species distribution and evolutionary adaptation—that can allow plant populations to adjust to climate change (Nicotra et al. 2010; Franks et al. 2014; Parmesan and Hanley 2015). Phenotypic plasticity is defined as the ability of a single genotype to express different phenotypes under different environmental conditions (Franks et al. 2014). Plasticity of various plant traits, such as plant height, leaf size, specific leaf area, and seed size and number, is considered to be important in species responses to climate change (Nicotra et al. 2010). However, the plasticity of certain regeneration traits, such as seed germination and seedling growth, is highly unknown, despite the critical role of early life-history stages in plant population persistence (Walck et al. 2011; Parmesan and Hanley 2015).
Plastic response of an individual to environmental conditions can be expressed not only in its own phenotype (within-generation phenotypic plasticity). Maternal environmental effect (or transgenerational phenotypic plasticity) refers to the phenomenon when the ecological environment experienced by the mother plant influences the offspring’s phenotype independently of the genetic inheritance of causative alleles (Roach and Wulff 1987; Herman and Sultan 2011). It can be mediated by multiple, often interacting mechanisms; for instance, changes in seed provisioning (i.e., the allocation of nutritive reserves to the developing seed), seed hormone content, or epigenetic marks (such as DNA methylation; Herman and Sultan 2011). The potential importance of transgenerational plasticity in plant species’ responses to global environmental changes is highlighted by an increasing number of studies (e.g., Hovenden et al. 2008; Pías et al. 2010; Schuler and Orrock 2012; Fenesi et al. 2014; Walter et al. 2016). If the progeny environment is reliably predictable from the maternal environment—e.g., for species with short-distance seed dispersal (Galloway and Etterson 2007)—the mother can adjust the phenotype of her offspring to enhance its performance under conditions that it is likely to encounter (Agrawal et al. 1999; Sultan et al. 2009; Herman and Sultan 2011; Fenesi et al. 2014). However, when increased stochasticity in temperature and/or precipitation associated with climate change decreases the reliability of environmental cues, transgenerational effects could reduce offspring performance (Schuler and Orrock 2012). Climate change experiments in natural vegetation have shown that rainfall manipulations in the maternal environment could influence various traits of offspring including seed germination and viability, seedling growth, or leaf C:N ratio. However, most of these studies focused on dominant species (Breen and Richards 2008; Pías et al. 2010; Tielbörger and Petrů 2010; Chamorro et al. 2016; Walter et al. 2016), and little research addressed the responses of other coexisting species (e.g., Li et al. 2011).
In semiarid regions, ecosystems on sandy soils can be particularly sensitive to precipitation changes, partly due to the low water-holding capacity of the soil (Yang et al. 2010; Gao et al. 2015; Huang et al. 2017). This is also the case in the open perennial sand grassland component of the Pannonian sand forest steppe in Hungary (Kovács-Láng et al. 2000). For example, extreme droughts in 2000 and 2003 resulted in a marked drop in the cover of the dominant perennial grasses, with a concomitant increase in the abundance of previously subdominant or subordinate annuals in these grasslands in the Danube–Tisza Interfluve, Central Hungary (Kovács-Láng et al. 2006). With a higher probability of drought in summer projected for the country (Bartholy et al. 2014), such annual-dominated patches may persist. The aims of this study were to assess (1) how the altered environment associated with field-scale experimental rainfall manipulations in a perennial sand grassland affect the growth, seed production, and seed mass of the characteristic subordinate winter annual grass Secale sylvestre, and (2) whether changes in the maternal environment caused by rainfall treatments influence the seed germination and offspring growth of this species in a subsequent pot experiment. We hypothesized that (H1) plants growing in the experimental plots (mother plants) show plasticity in the studied traits in response to the different environment resulting from rainfall treatments; (H2) the effect of maternal environment manifests in the offspring generation.
Materials and methods
Study site and rainfall manipulation experiment
The study site is located in the Danube–Tisza Interfluve, near the village Fülöpháza (46°52′N, 19°25′E) in the Kiskunság National Park. The climate is moderately warm semiarid temperate with continental and sub-Mediterranean influences. Annual mean temperature is 10.4 °C, and yearly average precipitation is 500–550 mm (1961–1990; Kovács-Láng et al. 2000). Midsummer drought is typical in July and August, and it is amplified by the coarse-textured calcareous sand soil.
The species selected for our study, Secale sylvestre Host is one of the most frequent winter annual grasses in open sand grasslands of the area. It is a characteristic subordinate component of perennial grasslands, but may become abundant on bare soils as a colonizer during secondary succession after disturbance (Kovács-Láng et al. 2000; Molnár 2003).
In 2015, we set up an experiment in an open sand grassland characterised by the dominance of two perennial bunchgrasses, Festuca vaginata Waldst. and Kit. ex Willd. and Stipa borysthenica Klokov ex Prokudin. Experimental units were 3 m × 3 m plots with a 50 cm buffer strip along each side inside the boundaries of each plot, and thus, the effective sampling area was 2 m × 2 m. Plots were laid out in a completely randomized block design with three treatments and a control (ambient rainfall), in six replicates (six blocks, each block containing one plot of each treatment). Treatments were as follows: severe drought from late June to late August (ca. 2 months), moderate drought from late July to late August (ca. 1 month), and watering as one event of ca. 25 mm per month from late May to late August (i.e., 100 mm per year, ca. 20% increase over the long-term annual mean; for exact dates, see Table 1). Thus, at the beginning of this study on S. sylvestre (April 2016), treatment plots had received 1 year of rainfall manipulations (in 2015). Treatments were repeated also in 2016 with a similar timing, but S. sylvestre plants studied received only the first watering treatment in late May before completing their life cycle in early June.
Drought treatments were conducted by excluding rain from the plots using permanent, transparent plastic foils. Watering treatment was applied using spraying heads at 1 m height, in a 1 m × 1 m grid. Side curtains were used during both treatments to avoid water addition to the plots neighbouring watered plots or prevent rain coming from the side to drought plots.
Air temperature at 20-cm height and volumetric soil water content (SWC, %) at 0–30-cm depth (i.e., averaged over the entire soil profile up to 30 cm) were recorded in each plot by installed temperature and moisture sensors (Sensirion SHT75 and Campbell CS616, respectively) connected to a data logger. Precipitation was measured with rain gauges (Davis DS7852) at 30 cm.
Background conditions for the studied S. sylvestre plants: precipitation, soil water content, and plant abundance
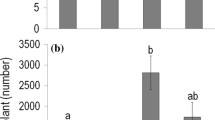
Severe and moderate drought treatments excluded 62% and 49% of ambient rainfall, respectively, while watered plots received 50% more rainfall than control plots between 1 May and 31 August 2015 (Table 1). During this 4-month period, on average, rain exclusions decreased soil water content from 4.0% (control) to 3.2% and 3.4% in severe and moderate drought plots, respectively, whereas watering treatment increased average SWC to 4.3%. Severe drought treatment in summer 2015 decreased the cover of F. vaginata and S. borysthenica by ca. 80% by April 2016 compared to April 2015, while, in watered plots, the cover of these perennial grasses increased by 20% (Table 1). As a result, in April 2016, the abundance of the two dominant perennial grasses in severe drought plots was 83% lower than in control plots, and 87% lower than in watered plots. On the contrary, the cover of S. sylvestre increased almost fivefold in severe drought plots from April 2015 to April 2016, which led to eightfold and fourfold higher abundance of this grass in these plots than in watered and control plots, respectively, in April 2016.
Field sampling and data collection
In April 2016, ten individuals were selected and marked for repeated measurements within the 4-m2 sampling area of each plot. We measured the maximum (vegetative) shoot height (stretched length of the shoot; accuracy 0.5 cm) according to the protocol of Cornelissen et al. (2003). The length of ear without arista was measured in the early June, in the ripening phase, when caryopses (referred to as seeds hereafter) had reached their final size, but were not yet loosening (Lancashire et al. 1991). For the few individuals that developed tiller(s), the longest shoot and ear were chosen. Seed number per ear was estimated using a linear regression equation (r2 = 0.98, P < 0.0001) between the length and seed number of ears determined on an additional 30 individuals outside, but close to the experimental plots at the same date. Fully ripened seeds from 15 to 20 randomly selected individuals per plot were collected on 9 June 2016, when most of the S. sylvestre plants had completed their life cycle. Seeds were stored in paper bags at room temperature (ca. 28 °C in summer and 15 °C in winter) until used for the germination experiment. Fifty “apparently viable” seeds per plot (i.e., that appeared to be intact and resisted gentle pressure; Roberts 1981) were weighed individually (accuracy 0.1 mg) to determine mean single seed mass.
Germination and growth experiment
To examine the effects of maternal environment on offspring germination and growth, a common garden pot experiment was set up in Fót (47°37′N, 19°10′E), ca. 83 km from the field site, on 16 March 2017. In this experiment, four half-litre pots were used to represent one experimental field plot. Thus, 96 pots in total (four treatments, six blocks) filled with nutrient-poor sandy soil were placed onto the bench of an outdoor, open-air growth facility. Pots were exposed to natural weather conditions except for excluding precipitation by a transparent plexiglass roof. From each plot of the field experiment, 36 seeds were sown in four pots (nine seeds per pot). Final percentage of germination (i.e., coleoptile emerged ≥ 2 mm above the soil surface) was determined after 35 days. Seedlings were thinned to the largest (≥ 5 cm) one per pot, and were grown under well-watered conditions (as the major growth period of this grass (April–May) is usually not water-limited). Pots were rotated weekly on the bench to minimize the micro-environmental differences associated with pot position. Until the end of July, when shoot biomass was harvested, only 11 individuals entered the reproductive phase, and 85 plants remained vegetative (most likely due to the lack of exposure to chilling required for flowering; Chouard 1960). Shoot height was measured at two life stages: for 3-week-old plants (juveniles, which had two fully expanded leaves), and for 4-month-old plants (referred to as adults). In addition, total leaf number and the length of fully expanded leaves were determined at juvenile stage. Juvenile shoot size was calculated by multiplying the total number of leaves by the length of the longest fully expanded leaf. This index is frequently used as a non-destructive estimate for biomass, particularly of juvenile plants (e.g., Van Groenendael and Slim 1988; Vergeer and Kunin 2013). It showed strong correlation with juvenile shoot biomass also for S. sylvestre (Pearson’s r = 0.90, P < 0.0001), measured on an additional 30 3-week-old plants in a separate experiment. Green (live) biomass was harvested from 4-month-old adult plants (referred to as adult biomass), oven-dried at 60 °C for 48 h, and weighed. Reproductive adults and those that died during the experiment (four plants) were excluded from data collection at adult stage. Thus, in the growth experiment, 1–4 individuals (pots) corresponded to a single treatment plot of the field experiment.
Statistical analysis
For each plant response variable, statistical analyses were done on mean values per plot as the experimental unit (n = 6). General linear mixed models with treatment as a fixed effect and block as a random factor were conducted for maximum shoot height, seed number per ear, and mean single seed mass of maternal generation. Data met the assumptions of normality of residuals and homoscedasticity (Quinn and Keough 2002). For post hoc comparison of means, Tukey’s HSD tests were used. To assess the effect of shoot height on seed number and seed mass after controlling for the effect of treatments, shoot height was also included in the model as a continuous predictor variable, and the partial correlation coefficients (R) were calculated.
For monthly average SWC during the growth and reproduction of the studied plants, two-way repeated-measures ANOVA was used with treatment as a fixed effect and month as the repeated-measures effect. Subsequently, Tukey’s HSD tests were applied between treatments within each month separately. For each analysis, the TIBCO Statistica software (TIBCO Software Inc. 2017) was used, and differences were considered significant at P < 0.05.
Results
Maternal generation
During the peak growth period of the maternal generation of S. sylvestre, soil water content was higher in severe drought plots than in both watered and control plots in May 2016 (severe drought and watered plots differed also in April with marginal significance: P = 0.091; Fig. 1).
Effects of rainfall manipulations on volumetric soil water content (%) at 0–30-cm depth in the plots of the field experiment during the period of growth and reproduction of the studied Secale sylvestre plants (maternal generation). Values are treatment mean ± SE (n = 6) in each month. Treatments are watering (W), control (C), moderate drought (M), and severe drought (S). Different letters above the bars denote significant (P < 0.05) differences, while N.S. indicates the lack of significant differences between treatments within each month separately. Results of Tukey’s HSD tests following two-way repeated-measures ANOVA are shown
Rainfall manipulations had a significant effect on each plant response variable studied in S. sylvestre growing in the plots of the field experiment (Table 2). Plants growing in severe drought plots had both higher maximum vegetative shoot height and higher seed number per ear than those growing in control and watered plots (Fig. 2a, b). Consistently, individuals growing in moderate drought plots also showed higher values than those in watered plots. Mean single seed mass was greater in severe drought plots than in control and the other treatment plots (Fig. 2c). Difference between the highest and the lowest treatment means (severe drought and watering, respectively), expressed as percentage of the lowest mean ([(max–min)/min] × 100, %), was 3.7 times higher in seed number (68.9%) than in seed mass (18.6%). However, when controlling for the effect of treatments, both these components of reproductive success showed a strong positive partial correlation with shoot height (R = 0.89, P < 0.0001 for seed number; R = 0.74, P = 0.0012 for seed mass).
Effects of the previous-year (2015) rainfall manipulations on a maximum vegetative shoot height (cm), b seed number per ear, and c mean single seed mass (mg) of Secale sylvestre growing in the plots of the field experiment in 2016 (maternal generation). Values are treatment mean ± SE (n = 6). Treatment symbols are defined in the legend of Fig. 1. Different letters above the bars indicate significant (P < 0.05) differences between treatments. Results of Tukey’s HSD tests following general linear mixed models are shown
Offspring generation
Seeds produced in control and rainfall manipulated plots did not differ in final germination percentage (67–80%); only a marginally significant difference (P = 0.086) was found between watering and moderate drought treatments; Table 2; Fig. 3a). However, maternal environment had significant effects on the three-week-old offspring (Table 2; Fig. 3b–d). Both juvenile shoot size and the length of the first fully expanded leaf were higher for the offspring whose mother plants grew in severe or moderate drought plots than for the progeny whose mothers developed in watered plots (Fig. 3b, c). Similar differences were found in juvenile shoot height, though severe drought and watering treatments differed with only marginal significance (P = 0.058; Fig. 3d). At the time of harvest, neither shoot height nor shoot biomass of the adult progeny varied significantly with the environment of their mothers (Table 2; Fig. 3e, f).
Effects of differences in the maternal environment resulting from rainfall manipulations (which were applied in 2015) on Secale sylvestre (offspring generation), whose mothers grew in the plots of the field experiment in 2016. Offspring were grown in a subsequent common garden pot experiment. Plant response variables include a final germination percentage (%), b juvenile shoot size calculated by multiplying the total number of leaves by the length of the longest fully expanded leaf (cm), c length of the first fully expanded leaf (cm), d juvenile shoot height (cm), e adult shoot height (cm), and f adult live biomass (mg). Values are treatment mean ± SE (n = 6). The treatments of the field experiment are abbreviated as in Fig. 1. The statistical tests applied and the indication of significant (P < 0.05) differences between the treatments are the same as described in Fig. 2
Discussion
Plasticity of maternal generation
Shoot growth and seed production of the studied S. sylvestre plants provided evidence in favour of H1, which was that S. sylvestre growing in the experimental plots exhibited phenotypic plasticity in the studied traits in response to the different environment caused by rainfall manipulations. The positive relationships of both seed mass and number with shoot height indicate that, on average at plot level, plants experiencing a better resource availability can allocate more assimilate to both vegetative growth and reproduction. Lower variation in seed mass than in number is consistent with the previous consideration that seed size is often the least plastic component of reproductive yield within a species (Harper et al. 1970). Nevertheless, S. sylvestre has a limited seed dispersal capacity, and most seeds fall beneath the mother plant. For such species, density-dependent mortality can be high, e.g., due to intense competition between progeny seedlings, and thus, larger maternal plants may benefit from producing larger seeds (Venable 1992). The seed number and mass values obtained across treatments in our study were within or close to the wide range reported for these traits in several populations of this species within the Kiskunság region (i.e., 5.3–7.9 g for thousand seed mass, and 12 and 26 as a minimum and maximum number of grains per ear, respectively; Vörösváry et al. 2000).
In several other water manipulation experiments in arid and semiarid ecosystems, reduction in the amount of rainfall usually limited plant growth and/or seed production, whereas increased water supply had an opposite effect (e.g., Poulin et al. 2007; Breen and Richards 2008; Gao et al. 2015; Volis et al. 2015). In contrast, S. sylvestre in our study, showed enhanced growth and reproductive performance in the experimental plots exposed to 2-month drought in the previous year, particularly compared with the individuals growing in plots that received supplemental watering. The most probable explanation for these apparently contradictory results is that S. sylvestre was not impacted directly by dry conditions (either in 2015 or 2016), because this grass usually completes its life cycle in early June, i.e., before the start of severe drought treatment in late June (Table 1). This phenological rhythm is typical for winter annual species in sand grasslands (Kárpáti and Kárpáti 1954). However, this subordinate species might have benefited indirectly from rain exclusion, as severe drought treatment in 2015 negatively affected the abundance (and thus the competitive effect) of the two dominant perennial grasses, which did not recover by April 2016 (Table 1). This likely resulted in a better resource (particularly water) availability for S. sylvestre during its peak growth period in spring 2016. In contrast, the moderate increase in the cover of dominant perennials in response to watering during summer 2015 (Table 1) might have enhanced the suppression of the coexisting annual S. sylvestre in spring 2016. This interpretation is supported by the higher soil water content in severe drought plots compared to watered plots in April and particularly in May 2016 (Fig. 1), most likely due to the lower transpirational water loss of the decreased perennial grass cover.
Our results also suggest that, with recurring severe droughts, the higher abundance of S. sylvestre in severe drought plots in April 2016 after a single 2-month drought of the previous summer (Table 1) may be further augmented by the enhanced growth and reproductive capacity of this annual grass due to the negative response of the concurrent dominant perennial grasses to drought. Similar to our results, in a California grassland, experimentally extended spring rainfall imposed limited direct effects on winter annual grasses due to their early phenology, but, in the subsequent year, these grasses benefited indirectly from the decomposition of the initially expanding N-fixing forbs (Suttle et al. 2007). Our results are also in line with those of Violle et al. (2006), who reported that the experimental removal of standing biomass of the dominant perennial grass (with the retention of litter) enhanced the total final and the seed biomass per plant of two early successional annual species in an old field.
In our study, S. sylvestre in watered plots was directly exposed to watering in May both in 2015 and 2016, during flowering. However, the competitive effect of the dominant perennial grasses might have overridden the potential direct positive impact of supplemental water on the annual S. sylvestre. Similarly, in another water manipulation experiment in a mountain steppe, Liancourt et al. (2013) demonstrated that the negative effects of competition with neighbouring plants (including dominant species) could offset the direct benefit of added water on the above-ground biomass of a characteristic species. However, the net effect of supplemental rainfall may depend on how strongly precipitation change alters competition, and also on the sensitivity of inferior species to the altered competitor abundance (Levine et al. 2010).
Plasticity of offspring generation
In agreement with H2, which was that differences in the maternal environment caused by rainfall manipulations affected the offspring generation of S. sylvestre, we found plasticity in the growth of progeny at the juvenile stage. In contrast, seed germination percentage and the adult growth of offspring were not influenced by the environmental conditions of their mother plants. When seed dormancy is imposed by biochemical constraints, drought during seed development usually decreases dormancy and increases germinability (Fenner 1991), which has also been demonstrated in some recent rainfall manipulation experiments with annual species (Karimmojeni et al. 2014; Gao et al. 2015). Nevertheless, some other studies reported similar or higher germination percentage in response to better water conditions in the maternal environment (Poulin et al. 2007; Breen and Richards 2008; Pías et al. 2010; Li et al. 2011).
In the juvenile phase, the size of both the first leaf and the whole shoot was greater for the progeny whose mothers grew in drought plots compared with the offspring whose mothers developed in watered plots. This indicates that mother plants experiencing less competitive and thus more favourable (moisture) environment (i.e., in severe and moderate drought plots, where the cover of dominant perennial grasses was low; Table 1) facilitated the early growth of their offspring. Larger plant size in the early phase of the life cycle might provide a great advantage for survival, as mortality rate of young plants is often high (Leishman et al. 2000), and can be size-dependent within a species, especially in resource-limited conditions, such as under water stress (Cook 1980; Parker 1982). Such a positive maternal effect can allow offspring to avoid the initial time lag that is required for the development of the offspring’s own plasticity to its actual environment (Agrawal et al. 1999; Herman and Sultan 2011). Numerous prior studies reported that a better water availability for the studied species in the maternal environment had positive transgenerational effects on offspring growth in the early seedling or juvenile stage, i.e., in the phase that can be critical for establishment (Breen and Richards 2008; Pías et al. 2010; Li et al. 2011; Walter et al. 2016). Larger seedlings usually germinate from larger seeds, and greater seed mass often reflects a higher amount of seed reserves (Leishman et al. 2000). In our experiment, greater mass was detected only for seeds produced in severe drought plots, and thus, other potential mechanisms than seed provisioning (reviewed by Herman and Sultan 2011) should (also) account for the differences in juvenile growth observed between the progeny whose mother plants grew in severe or moderate drought plots and in watered plots.
We found no difference in shoot height and biomass of 4-month-old progeny according to the environment of their mothers. These results are consistent with the previous studies, reporting that the beneficial maternal effects diminished or disappeared in a later stage of offspring’s life cycle (Pías et al. 2010; Walter et al. 2016), but contrast with the other studies where positive transgenerational effect was detected in the final fitness of adult progeny or both in an earlier and adult stages (Roach and Wulff 1987; Fenesi et al. 2014). The persistence of positive maternal influence may depend on its underlying mechanism (Herman and Sultan 2011), and also on the environmental conditions experienced by the offspring. For example, the improved seedling vigour of Austrian winter field peas established from large seeds could increase the seed yield compared to the yield of peas planted from small seeds under adverse conditions, but not in environment more favourable for pea growth at Grangeville, Idaho (Murray et al. 1984). Thus, the fact that, in our experiment, the progeny of S. sylvestre were grown under well-watered conditions might provide one possible explanation why the benefit of enhanced growth of juveniles did not appear in the adult stage. Nevertheless, to our best knowledge, our study provides the first experimental evidence that altered rainfall amounts, this key element of climate change, can trigger transgenerational effects on offspring growth of a subordinate species indirectly via changes in the competitive interactions with the dominant species.
Conclusions
Our field experiment showed that a subordinate species in perennial sand grasslands, S. sylvestre, exhibited phenotypic plasticity in shoot growth and seed production when growing in different environments caused by a single year of rainfall manipulations. This plasticity is most likely a response to the altered population interactions in the growth environment resulting from the previous-year precipitation changes, which led to enhanced performance of this species with decreasing amount of rainfall. Moreover, maternal environmental effect found in the early growth of offspring might amplify the immediate response that can be achieved by within-generation plasticity alone (Sultan et al. 2009; Herman and Sultan 2011). Based on these results, we expect that summer drying projected for Hungary in the future (Bartholy et al. 2014) will favour the growth and reproduction of S. sylvestre. This better performance may contribute to the increase in abundance of this annual grass, and, thus, to the shift from perennial grasses to annuals in sand grasslands of the study region. Our study highlights that both within-generation and transgenerational plasticity of subordinate species should be taken into account to better understand and predict shifts in plant species or functional group abundances under climate change.
References
Agrawal AA, Laforsch C, Tollrian R (1999) Transgenerational induction of defences in animals and plants. Nature 401:60–63. https://doi.org/10.1038/43425
Bai Y, Han X, Wu J, Chen Z, Li L (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184. https://doi.org/10.1038/nature02850
Bartholy J, Pongrácz R, Pieczka I (2014) How the climate will change in this century? Hung Geogr Bull 63:55–67. https://doi.org/10.15201/hungeobull.63.1.5
Breen AN, Richards JH (2008) Irrigation and fertilization effects on seed number, size, germination and seedling growth: implications for desert shrub establishment. Oecologia 157:13–19. https://doi.org/10.1007/s00442-008-1049-3
Chamorro D, Parra A, Moreno JM (2016) Reproductive output, seed anatomy and germination under water stress in the seeder Cistus ladanifer subjected to experimental drought. Environ Exp Bot 123:59–67. https://doi.org/10.1016/j.envexpbot.2015.11.002
Chouard P (1960) Vernalization and its relations to dormancy. Annu Rev Plant Physiol 11:191–238. https://doi.org/10.1146/annurev.pp.11.060160.001203
Cook RE (1980) Germination and size-dependent mortality in Viola blanda. Oecologia 47:115–117. https://doi.org/10.1007/BF00541785
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchman N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
Dudney J, Hallett LM, Larios L, Farrer EC, Spotswood EN, Stein C, Suding KN (2017) Lagging behind: have we overlooked previous-year rainfall effects in annual grasslands? J Ecol 105:484–495. https://doi.org/10.1111/1365-2745.12671
Fenesi A, Dyer AR, Geréd J, Sándor D, Ruprecht E (2014) Can transgenerational plasticity contribute to the invasion success of annual plant species? Oecologia 176:95–106. https://doi.org/10.1007/s00442-014-2994-7
Fenner M (1991) The effects of the parent environment on seed germinability. Seed Sci Res 1:75–84. https://doi.org/10.1017/S0960258500000696
Franks SJ, Weber JJ, Aitken SN (2014) Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl 7:123–139. https://doi.org/10.1111/eva.12112
Galloway LF, Etterson JR (2007) Transgenerational plasticity is adaptive in the wild. Science 318:1134–1136. https://doi.org/10.1126/science.1148766
Gao R, Yang X, Liu G, Huang Z, Walck JL (2015) Effects of rainfall pattern on the growth and fecundity of a dominant dune annual in a semi-arid ecosystem. Plant Soil 389:335–347. https://doi.org/10.1007/s11104-014-2366-4
Harper JL, Lovell PH, Moore KG (1970) The shapes and sizes of seeds. Annu Rev Ecol Syst 1:327–356. https://doi.org/10.1146/annurev.es.01.110170.001551
Herman JJ, Sultan SE (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front Plant Sci 2:1–10. https://doi.org/10.3389/fpls.2011.00102
Hovenden MJ, Wills KE, Chaplin RE, Vander Schoor JK, Williams AL, Osanai YUI, Newton PC (2008) Warming and elevated CO2 affect the relationship between seed mass, germinability and seedling growth in Austrodanthonia caespitosa, a dominant Australian grass. Glob Change Biol 14:1633–1641. https://doi.org/10.1111/j.1365-2486.2008.01597.x
Huang Y, Yu X, Li E, Chen H, Li L, Wu X, Li X (2017) A process-based water balance model for semi-arid ecosystems: a case study of psammophytic ecosystems in Mu Us Sandland, Inner Mongolia, China. Ecol Model 353:77–85. https://doi.org/10.1016/j.ecolmodel.2017.01.005
Kardol P, Campany CE, Souza L, Norby RJ, Weltzin JF, Classen AT (2010) Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Glob Change Biol 16:2676–2687. https://doi.org/10.1111/j.1365-2486.2010.02162.x
Karimmojeni H, Bazrafshan AH, Majidi MM, Torabian S, Rashidi B (2014) Effect of maternal nitrogen and drought stress on seed dormancy and germinability of Amaranthus retroflexus. Plant Species Biol 29:e1–e8. https://doi.org/10.1111/1442-1984.12022
Kárpáti I, Kárpáti V (1954) The aspects of the calciphilous turf (Festucetum vaginatae danubiale) in the environs of Vácrátót in 1952. Acta Bot Acad Sci Hung 1:129–157
Kovács-Láng E, Kröel-Dulay Gy, Kertész M, Fekete G, Bartha S, Mika J, Dobi-Wantuch I, Rédei T, Rajkai K, Hahn I (2000) Changes in the composition of sand grasslands along a climatic gradient in Hungary and implications for climate change. Phytocoenologia 30:385–407. https://doi.org/10.1127/phyto/30/2000/385
Kovács-Láng E, Kröel-Dulay Gy, Rédei T, Lhotsky B, Garadnai J (2006) The effect of climate change on forest-steppe ecosystems in the Carpathian Basin. In: Láng I, Faragó T, Iványi Zs (eds) International conference on climate change: impacts and responses in Central and Eastern European countries, Pécs, 5–8 November 2005, pp 294–300
Lancashire PD, Bleiholder H, van Den Boom T, Langelüddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601. https://doi.org/10.1111/j.1744-7348.1991.tb04895.x
Leishman MR, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. CAB International, Wallingford, pp 31–57
Levine JM, McEachern AK, Cowan C (2010) Do competitors modulate rare plant response to precipitation change? Ecology 91:130–140. https://doi.org/10.1890/08-2039.1
Li Y, Yang H, Xia J, Zhang W, Wan S, Li L (2011) Effects of increased nitrogen deposition and precipitation on seed and seedling production of Potentilla tanacetifolia in a temperate steppe ecosystem. PLoS One 6:e28601. https://doi.org/10.1371/journal.pone.0028601
Liancourt P, Spence LA, Song DS, Lkhagva A, Sharkhuu A, Boldgiv B, Helliker BR, Petraitis PS, Casper BB (2013) Plant response to climate change varies with topography, interactions with neighbors, and ecotype. Ecology 94:444–453. https://doi.org/10.1890/12-0780.1
Mariotte P (2014) Do subordinate species punch above their weight? Evidence from above- and below-ground. New Phytol 203:16–21. https://doi.org/10.1111/nph.12789
Mariotte P, Vandenberghe C, Kardol P, Hagedorn F, Buttler A (2013) Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J Ecol 101:763–773. https://doi.org/10.1111/1365-2745.12064
Molnár Zs (ed) (2003) A Kiskunság száraz homoki növényzete. (Dry sand vegetation of the Kiskunság). TermészetBÚVÁR Alapítvány Kiadó, Budapest (in Hungarian with English translation)
Murray GA, Swensen JB, Auld DL (1984) Influence of seed size and planting date on the performance of Austrian winter field peas. Agron J 76:595–598. https://doi.org/10.2134/agronj1984.00021962007600040021x
Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692. https://doi.org/10.1016/j.tplants.2010.09.008
Parker MA (1982) Association with mature plants protects seedlings from predation in an arid grassland shrub, Gutierrezia microcephala. Oecologia 53:276–280. https://doi.org/10.1007/BF00545677
Parmesan C, Hanley ME (2015) Plants and climate change: complexities and surprises. Ann Bot 116:849–864. https://doi.org/10.1093/aob/mcv169
Pías B, Matesanz S, Herrero A, Gimeno TE, Escudero A, Valladares F (2010) Transgenerational effects of three global change drivers on an endemic Mediterranean plant. Oikos 119:1435–1444. https://doi.org/10.1111/j.1600-0706.2010.18232.x
Poulin J, Sakai AK, Weller SG, Nguyen T (2007) Phenotypic plasticity, precipitation, and invasiveness in the fire-promoting grass Pennisetum setaceum (Poaceae). Am J Bot 94:533–541. https://doi.org/10.3732/ajb.94.4.533
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, New York
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235. https://doi.org/10.1146/annurev.es.18.110187.001233
Roberts HA (1981) Seed banks in soils. Adv Appl Biol 6:1–55
Sala OE, Parton WJ, Joyce LA, Lauenroth WK (1988) Primary production of the central grassland region of the United States. Ecology 69:40–45. https://doi.org/10.2307/1943158
Schuler MS, Orrock JL (2012) The maladaptive significance of maternal effects for plants in anthropogenically modified environments. Evol Ecol 26:475–481. https://doi.org/10.1007/s10682-011-9499-1
Scott RL, Hamerlynck EP, Jenerette GD, Moran M, Barron-Gafford GA (2010) Carbon dioxide exchange in a semidesert grassland through drought-induced vegetation change. J Geophys Res 115:G03026. https://doi.org/10.1029/2010JG001348
Seddon AW, Macias-Fauria M, Long PR, Benz D, Willis KJ (2016) Sensitivity of global terrestrial ecosystems to climate variability. Nature 531:229–232. https://doi.org/10.1038/nature16986
Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–3289. https://doi.org/10.1890/08-1815.1
Sultan SE, Barton K, Wilczek AM (2009) Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90:1831–1839. https://doi.org/10.1890/08-1064.1
Suttle KB, Thomsen MA, Power ME (2007) Species interactions reverse grassland responses to changing climate. Science 315:640–642. https://doi.org/10.1126/science.1136401
TIBCO Software Inc. (2017) Statistica (data analysis software system), version 13 (trial version). http://statistica.io. Accessed 16 Dec 2017
Tielbörger K, Petrů M (2010) An experimental test for effects of the maternal environment on delayed germination. J Ecol 98:1216–1223. https://doi.org/10.1111/j.1365-2745.2010.01682.x
Van Groenendael JM, Slim P (1988) The contrasting dynamics of two populations of Plantago lanceolata classified by age and size. J Ecol 76:585–599. https://doi.org/10.2307/2260614
Venable DL (1992) Size-number trade-offs and the variation of seed size with plant resource status. Am Nat 140:287–304. https://doi.org/10.1086/285413
Vergeer P, Kunin WE (2013) Adaptation at range margins: common garden trials and the performance of Arabidopsis lyrata across its northwestern European range. New Phytol 197:989–1001. https://doi.org/10.1111/nph.12060
Violle C, Richarte J, Navas ML (2006) Effects of litter and standing biomass on growth and reproduction of two annual species in a Mediterranean old-field. J Ecol 94:196–205. https://doi.org/10.1111/j.1365-2745.2005.01061.x
Volis S, Ormanbekova D, Yermekbayev K (2015) Role of phenotypic plasticity and population differentiation in adaptation to novel environmental conditions. Ecol Evol 5:3818–3829. https://doi.org/10.1002/ece3.1607
Vörösváry G, Már I, Holly L, Kissimon J (2000) Analysis of genetic polymorphisms in jointed goatgrass (Aegilops cylindrica) and annual wild rye (Secale sylvestre) populations from Hungary. Port Acta Biol 19:137–147
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Glob Change Biol 17:2145–2161. https://doi.org/10.1111/j.1365-2486.2010.02368.x
Walker B, Kinzig A, Langridge J (1999) Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2:95–113. https://doi.org/10.1007/s100219900062
Walter J, Harter DE, Beierkuhnlein C, Jentsch A (2016) Transgenerational effects of extreme weather: perennial plant offspring show modified germination, growth and stoichiometry. J Ecol 104:1032–1040. https://doi.org/10.1111/1365-2745.12567
Yang HL, Huang ZY, Ye YZ, Zhu XW, Dong M, Weng HB (2010) Effects of soil moisture profile on seedling establishment in the psammophyte Hedysarum laeve in the semiarid Otindag Sandland, China. J Arid Environ 74:350–354. https://doi.org/10.1016/j.jaridenv.2009.09.014
Yang Z, Jiang L, Su F, Zhang Q, Xia J, Wan S (2016) Nighttime warming enhances drought resistance of plant communities in a temperate steppe. Sci Rep 6:23267. https://doi.org/10.1038/srep23267
Acknowledgements
This work is a part of the projects Nos. 120844 and 112576, which has been implemented with the support provided by the National Research, Development and Innovation Fund (NRDI Fund) of Hungary, financed under the PD_16 (AM) and K (GK-D) funding scheme, respectively. This study was also part of the project Sustainable Use of Ecosystem Services (GINOP-2.3.2-15-2016-00019) funded by the NRDI Office (GK-D and GÓ). This research was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (GK-D). We are grateful to the Kiskunság National Park for the support to our field work. We thank Péter Ódor for his advice on statistical analyses. We also thank the two anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Contributions
GK-D designed and established the rainfall manipulation experiment. AM and GK-D conceived the concept of the research. AM conducted fieldwork with the help of BL in developing the methodology. GÓ collected and processed the micrometeorological and vegetation cover data. AM, TK, and PC designed, and AM performed the pot experiment. AM analysed the data and wrote the manuscript with major inputs from all co-authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and/or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Carly Stevens.
Rights and permissions
About this article
Cite this article
Mojzes, A., Ónodi, G., Lhotsky, B. et al. Within-generation and transgenerational plasticity in growth and regeneration of a subordinate annual grass in a rainfall experiment. Oecologia 188, 1059–1068 (2018). https://doi.org/10.1007/s00442-018-4264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4264-6